Approach before alcoholic fermentation of mixtures with syrup in a Cuban distillery
Yailet Albernas Carvajal 1*, Yodalys Lamas Pérez 2, Ana Celia de Armas Martínez 3,
Irenia Gallardo Aguilar 4
1Departamento de Ingeniería Química, Facultad de Química y Farmacia, Universidad Central “Marta Abreu” de Las Villas, Santa Clara / Cuba; yailetac@uclv.edu.cu.
2Empresa Agroindustrial Azucarera “Heriberto Duquesne”, Remedios / Cuba; yodalys.lamas@duquesne.azcuba.cu.
3Empresa de Bebidas y Refrescos, Villa Clara / Santa Clara /Cuba; celiadearmasmartinez@gmail.com.
4 Departamento de Ingeniería Química, Facultad de Química y Farmacia, Universidad Central “Marta Abreu” de Las Villas, Santa Clara / Cuba; irenia@uclv.edu.cu.
* Correspondence: yailetac@uclv.edu.cu
ABSTRACT
Because of the competition with final molasses in different productions, it is necessary to look for other sources of sugar substrates to obtain ethanol. Streams derived from sugar production, such as final molasses, syrup, or filter juice, contain fermentable sugars, representing an opportunity for ethanol production. This work conducted a preliminary study in the alcoholic fermentation stage using a mixture of filter juice, molasses, and syrup. It also analyzed the feasibility of using syrup as a raw ferment material obtained from low-quality sugarcane. The experimental study was carried out using a 2k-1 experimental design, considering as variables: substrate (molasses or syrup), dilution agent (water and filter juice), and type of acid (H2SO4 and H3PO4), and the response variable was the alcoholic percentage obtained. Syrup, diluted with water using H3PO4, is a viable option when low-quality sugarcane is present, allowing the alcoholic degrees between 5.45 and 5.47%. With filter juice, alcoholic degrees between 5.22 and 5.30% were obtained, which are lower than in other studies with filter juices from sugarcane of adequate quality. The most influential variables were the dilution and acidifying agents in the statistical model obtained using Statgraphics Centurion XV 15.1.0.2 software.
Keywords: experiment design; mixture; fermentation; syrup; substrate.
INTRODUCTION
Bioethanol production is a promising solution to the challenge of carbon emissions from global fuel consumption. However, several obstacles must be overcome to fully exploit its potential, including low product yield, the food versus fuel factor, and feedstock logistics1.
According to data from Ethanol Market Report2, the liquid ethanol market is forecast to increase by USD 63.07 billion from 2022 to 2032. Ethanol is widely produced through biochemical (fermentation) and thermochemical (gasification) routes for its applications in diversified areas, e.g., cosmetics, food and beverages, pharmaceutical, and transportation sectors as a promising biofuel3. The leading bioethanol producers are the United States of America and Brazil, which use food crops, corn, and sugarcane juice as feedstock through biochemical routes4.
Most ethyl alcohol produced worldwide is fermented from sugar sources such as Saccharum officinarum sugarcane5. This contains readily fermentable sugars, which allow for an easy and economical ethanol production process. In countries such as Cuba, the molasses from the sugar production process is used as a carbon source, and the yeast Saccharomyces cerevisiae is used as the alcohol-producing microorganism6,7. With these two, combined with ammonium salts as sources of nitrogen and phosphorus and a pH adjusted with sulfuric acid in the equipment suitable for the process, it is possible to obtain satisfactory alcohol production 8,9, 10.
During alcoholic fermentation, contaminating bacteria compete with yeast for sugar and nutrients, causing a significant decrease in ethanol production; therefore, the lower the pH, the more the fermentation medium is protected against possible bacterial attack. Sulfuric acid (H2SO4) is the most commonly used to adjust the pH of the fermentation medium, although there are studies investigating the feasibility of using phosphoric acid (H3PO4) and nitric acid (HNO3)11.
Worldwide trends in ethanol production are leading to the use of substrates that are alternative to the traditional sugar cane and sugar beet molasses. Among them are the lower-quality juices that are processed with sugar. Research by 9, 12-17 addresses the use of other substrates for alcoholic fermentation, such as juices from the sugar process, bagasse hydrolysate, and stillage from alcohol distillation, offering great significance for the use of intermediate products from the sugar industry, which at the same time contribute to improving the sugar process 18, 19.
According to Martínez Y 20, the economic efficiency of ethanol production is strongly influenced by the availability, market prices, and destinations of the best use of raw materials. Therefore, several studies have been conducted using combined substrates such as juice from mud filters, diluted juices, clarified juices, B molasses, and final molasses in the fermentation stage19, 21, 22-23. Fabelo24 made significant contributions with his study on the modeling and optimization of the fermentation stage, using stillage and filter juice mixed with final molasses in different proportions. Mixtures of final molasses (0.299), filter juices (0.342), and hydrolyzed bagasse liquor with molasses (0.358) at 5.26% alcohol have also been used as a source of carbohydrates25, 26. These studies have shown that it is possible to reduce production costs by using mixtures of different substrates. For the mixture, as mentioned earlier, Morales25 demonstrates that it is possible to save 67 % of the molasses to be purchased in the non-harvest period and 22.73% of the water.
By redirecting 30 % of the juices from the filters, the sugar produced is reduced by 5 %; at the same time, the incorporation into the process of material with a high content of non-sugars, colloids, and microorganisms, which ultimately damages the quality of the sugar, is avoided27.
The extraction of these secondary juice streams for ethanol production allows for a reduction of harmful substances in the raw sugar production process, such as insoluble solids, polysaccharides, ash, and other impurities that hinder the evaporation, concentration, and crystallization stages of sugar, as well as contributing to water savings in the fermentation process since part of it is replaced by these juices. In addition, it leads to greater efficiency in the clarification stage of the sugar process, obtaining a higher quality sugar, a decrease in steam consumption, and an increase in the availability of excess bagasse19, 28-30.
An essential aspect that frequently occurs in Cuban sugar factories is that sometimes the sugar cane that arrives at the sugar mills does not have the appropriate or standard quality for the production of raw sugar (low sucrose content (Pol), high content of soluble solids other than sucrose (average is 10-16 %) and high levels of cane fiber (average is 11-16 %)), which can be caused by various reasons that are not the purpose of this work.
In view of this situation, an analysis is needed on what would be the most favorable scenario, whether to produce raw sugar or to produce only syrup and use it as a substrate to obtain ethanol.
The goal of this work is to carry out a study of alcoholic fermentation using a mixture of filter juices, molasses, and syrup from low-quality sugarcane.
Raw materials
For the study of mixtures in the fermentation stage, final molasses (MF), filter juice (JF), and syrup (M) are used as substrates, all of them coming from the process of obtaining raw sugar in the Cuban sugar industry. Initially, the substrates were characterized to know their conditions before they were used in the subsequent experiments.
Final Molasses
The final molasses is the liquid separated from the sucrose crystals by centrifugation. It is a dense and viscous syrup, separated from the final cooked mass itself and from which it is not possible to crystallize more sugar by conventional methods, given the inverted sugar and high viscosity. It contains approximately 45-55% sucrose, 20-30% glucose and fructose, 10-20% water3, and also has small amounts of mannose in stored molasses31, 7. In the present study, 600 ml were taken at 26 ºC from the final molasses used as raw material in the fermentation stage, which was stored in the sugar factory.
Filter juice
Filter juice is the intermediate stream obtained during the separation operations of the mill mud extracted from the clarified juice in the raw sugar manufacturing process 32, 33. Clarified filter juice has almost no unfermentable sugars, so all sugars are considered fermentable31. In this case, 800 ml of the juice obtained at the sugar factory's rotary vacuum filter outlet was taken at approximately 40 ºC and allowed to decant at room temperature for subsequent studies.
Syrup
Syrup is a liquid product resulting from the evaporation of clarified juices, by evaporation, of excess water in the evaporators without removing the sugar. This syrup is close to saturation point and has 55-65% dissolved solids. It contains high sugars (33% to 75%) in sucrose, glucose, and fructose34. For the study, the syrup was taken at the outlet of the third evaporator, approximately 50 ºC. The sample taken was 600 ml and was allowed to cool to room temperature for subsequent studies10.
It appears relatively similar to molasses, but its color is darker and almost black. It has a sweet taste, similar to licorice, but with a bitter touch. In turn, it has a high nutritional content of carbohydrates, B vitamins, minerals, and a low water content16.
Raw materials characterization
The substrates used are characterized based on the main components that develop the fermentation. In each case, they are analyzed in the distillery laboratory in question using standardized methods.
Fermentation conditions
Saccharomyces cerevisiae yeast is used in fermentation, the inoculum is prepared similarly to the conventional factory, and the final molasses is used as substrate24, 35. In the pre-fermentation process, the molasses is diluted with water in the dissolver until it reaches the desired brix to feed the preferments and the fermenters, this mixture being known as the cushion. Subsequently, the yeast culture is carried out in the preferments in three phases. In the first phase, 20% of the total volume of the mattress pre-fermenters is added from the dissolver, phosphoric acid is added to regulate the pH from 4 to 5, and the necessary amount of urea, phosphate, and yeast is also added. In the second phase, the cushion is added up to half of the total capacity, adding urea, phosphoric acid, and phosphate in the same proportions. Finally, the pre-fermenters are completely filled, finishing the brix in a range of 10-11, repeating the same dosage of the previously added compounds. Samples are taken every two hours, and when the brix measurement is half of the initial value, it is ready to be transferred to a fermenter, leaving 20% of the volume for seed in said pre-fermenters.
Fermentation is carried out in an anaerobic beaker of 1 L capacity at ambient temperature (28 ºC) for 10 hours because the brix had already dropped and remained constant. For process control, the Brix degree was determined during propagation and pre-fermentation every one hour and during fermentation every two hours. Brix was determined using an optical refractometer with ATC-Precisso, according to the approved Cuban standards36-38. The concentration of reducing sugars was determined by the Eynon-Lane method24, while the alcoholic strength was determined by pycnometry at 20 ºC. A Reischauer glass pycnometer is used to determine the density of the alcohol by weighing differences using a balance with a precision of 0.1 mg according to standard 39.
Experimental design
A 2k-1 type design of experiments with replication is carried out according to the distribution of Table 1. The following are considered as independent variables: primary substrate [final molasses (1) or syrup (-1)], dilution agent [water (1) and filter juice (-1)], and acidifying agent [sulfuric acid H2SO4 (1) and phosphoric acid H3PO4 (-1)]. The experiments take into account up to 100% of the total reducing sugars (TRS) of the substrates, and the alcoholic percentage is considered a response variable. Table 2 shows the quantities of each stream used in the experiments.
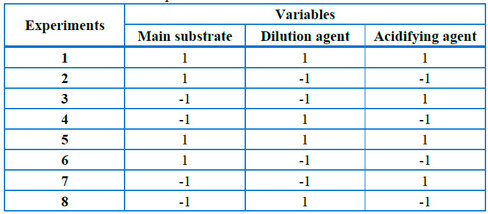
Table 1. Experiment planning

Table 2. Values of the streams in each experiment
Substrates characterization
Table 3 shows the main results obtained from characterizing the substrates used in the fermentation process.

Where RL: Light reductants, RT: Total reductants, AzT: Total sugars and AzF: Fermentable sugars.
Table 3. Main characteristics of the substrates used in fermentation
Fermentation results
The procedure applied in fermentation is similar to that developed in the distillery. First, a small bed of molasses is added, adding the volume of pre-fermenters needed to inoculate the pre-fermenters. The main characteristics of the pre-fermenters are shown in Table 4.

Table 4. Characteristics of the preferment used
Once the preferment is added to the diluted molasses bed, it is left to ferment under anaerobic conditions to ensure the transformation of sugars into alcohol and not to seek yeast propagation. The first step is carried out to gradually adapt the microorganism to the medium. The fermenter is filled when activity begins to be observed in the medium. The filling is carried out by refreshment, as in the industrial scale, considering the decrease of the concentration of total solids in the medium to a value equal to half plus one.
In the experimental fermentation study, the behavior of the Brix degrees was measured for each experiment (Figure 1). Samples were taken for analysis every hour until a constant value was reached (most of them after 6 hours), after which time cell death was expected to occur to determine the alcohol content (Figure 2). The time analyzed was 10 hours10.
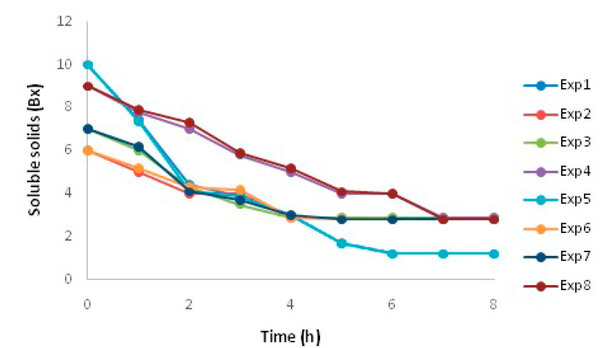
Figure 1. Brix behavior during fermentation
In all the variants, it was observed that there is consumption of substrates by the yeast, and no considerable alterations were perceived in indicators such as Brix; which indicates that the substrates used separately and in combination do not have inhibitory action due to the presence of salts, metabolites and other compounds. The variation of soluble solids in the fermented musts behaved similarly to the results achieved industrially and to those reported in studies carried out by authors such as 24, 16. In the first five hours of fermentation, a more than 44% decrease in soluble solids was observed in all the experiments, demonstrating substrate consumption at this stage.
The best results are achieved when only the final molasses is used (experiment 1), which supports the procedure applied in the factory when fermenting with this stream coming from the plant. While the experiments where the juice from the filters is used (2, 3 and their replies 6, 7), lower values (5.22-5.3 %) are obtained than those obtained by García15 (5.72), and Cortés40 (5.56). These results may be influenced by the quality of the cane from which the juice was extracted for fermentation. Figure 2 shows the alcoholic percentage and pH at the end of fermentation.
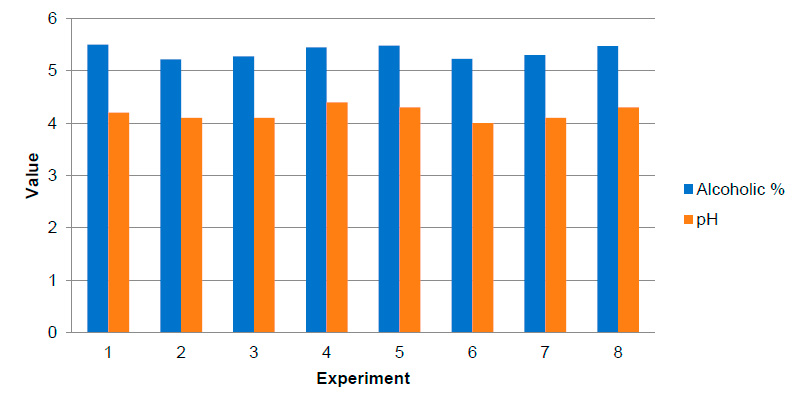
Figure 2. Alcoholic percentage and pH at the end of fermentation
The fermented musts have an alcoholic percentage between 5.22 and 5.50, these values are between the ranges obtained by Rivero19 [4.81 to 5.90 %], García15 [5.02 to 5.85 %] and Díaz16 [5.53 to 6.70 %], when they used filter juice, secondary juices and final molasses as substrates. When using final molasses in the fermentation diluted with water and H2SO4 as pH controller11, the range reached is 5.48 to 5.5 %, higher than that obtained by García15 (5.15 %) and equal to that of Cortés40 (5.5 %). This supports the traditional Cuban industrial process.
The best results were obtained in the experiments using the syrup diluted with water using H3PO4 (experiments 4 and 8). Alcoholic percentages between 5.45 and 5.47 % were obtained. The syrup is an attraction of the present work for low quality canes, confirming the viability of H3PO4 as an acidifying agent11.
The worst results, were obtained where the juice of the filters (2, 3 and their replicates 6, 7) was used when obtained in this study from low quality canes, ranges between 5.22 and 5.3 % are obtained, lower than those obtained by García15 (5.72 %) and Cortés40 (5.56 %). This was caused because these juices were extracted from low quality canes, this was discusses at the conference41.
The lowest pH value at the end of fermentation was reached when using the juice from the filters and the final molasses (experiment 2 and 6), which indicates that under these conditions there was a higher production of weak organic acids such as succinic and acetic acids, which can lower the pH of the culture medium, as explained by42. The pH obtained at the end of fermentation was similar to the values reported by43, 16, authors who likewise worked with molasses and sugarcane juice fermentations.
Statistical analysis of the experimental design
The statistical results were obtained by processing the experimental design in Statgraphics Centurion XV 15.1.0.2 software. By means of the regression study for the alcoholic percentage, the statistical model that adjusts to each of its coefficients was obtained, which is reported by equation (1). The R2 value reached indicates that the model thus adjusted explains 99.3525% of the variability in the alcoholic strength, there being a statistically significant relationship between the variables with a confidence level of 95.0%.
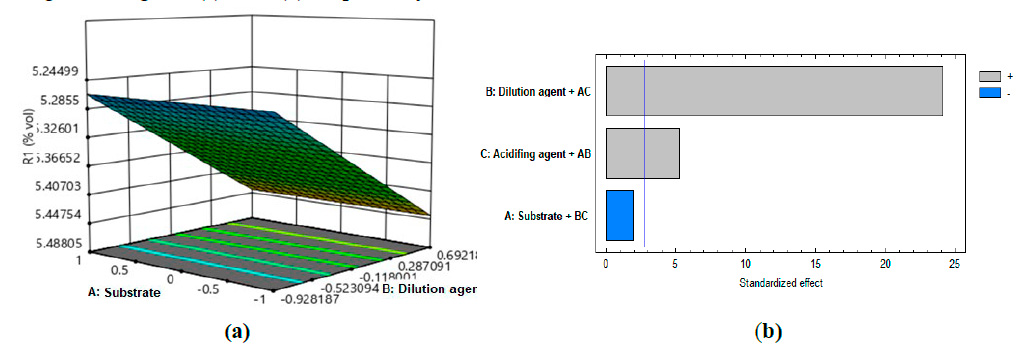
Figure 3. Response surface plot (a) and Pareto plot (b) for the results of the experimental design
It is observed that the most significant influence is exerted by the dilution agent, followed by the type of acid used to regulate the pH. In this case, the substrate does not show a significant influence from the qualitative point of view since it is used as a base to establish, at a constant value, the starting point of the soluble solids at the beginning of the fermentation.
In summary, the results of this study suggest that syrup, diluted with water and using H3PO4 as a pH control agent, is a viable option for ethanol production when using low-quality sugarcane. Alcohol degrees between 5.45 and 5.47% were obtained, indicating that this method may be an efficient alternative for utilizing sugarcane that is not optimal for sugar production. However, using filter juice in alcoholic fermentation is not recommended when the cane is of low quality, as lower alcohol degrees are obtained (between 5.22 and 5.30%) compared to other studies using filter juice from adequate-quality cane. The statistical analysis of the experimental design confirmed that the dilution and acidifying agents are the variables that most influence the alcoholic fermentation process.
CONCLUSIONS
This preliminary study on alcoholic fermentation using filter juice, molasses, and syrup as substrates has provided valuable information on the feasibility of using different sugar sources for ethanol production, especially in the Cuban sugar industry.
The results show that syrup, obtained from low-quality sugarcane, can be a viable alternative to final molasses in ethanol production, provided it is diluted with water, and H3PO4 is used to control pH. This finding is relevant to the Cuban sugar industry, as it allows sugarcane that does not meet the quality standards for sugar production.
On the other hand, filter juice is not recommended as a substrate for alcoholic fermentation when the sugarcane is of low quality since the alcohol degrees obtained are lower than those reported in previous studies with filter juice from good-quality sugarcane.
Statistical analysis of the 2k-1 experimental design confirmed the influence of the dilution agent and the type of acid used to regulate the pH on the final alcohol content.
This study offers an overview of the possibilities of diversifying sugar sources for ethanol production in the Cuban sugar industry, contributing to the search for sustainable and efficient resource-use alternatives.
Author Contributions: investigation, A.C.d.A., Y.A. and Y.L.; formal analysis, A.C.d.A., and Y.A.; writing original draft preparation, Y.A., A.C.d.A., and Y.L.; writing review and editing, Y.A., and A.C.d.A.; supervision, A.C.d.A. and Y.A.; project administration, A.C.d.A.; software, I.G and A.C.d.A. All authors have read and agreed to the published version of the manuscript.
Funding: This research was funded by the territorial project "Increase of high value products in the sugarcane industry using renewable energies", PT223VC004-003.
Data Availability Statement: Data are available through the authors.
Acknowledgments: The authors are grateful for the support provided by Empresa Agroindustrial Azucarera "Heriberto Duquesne".
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Aruwajoye G.S, Rorke D, Sanusi I.A, Sewsynker-Sukai Y, Gueguim E. Bioethanol Production Using Novel Starch Sources. Home Bioethanol: A Green Energy Substitute for Fossil Fuels; 2023. https://link.springer.com/chapter/10.1007/978-3-031-36542-3_5
2. Ethanol market report. Precedence Research. Chemical and material, 2023 (accessed on August 14, 2024). https://www.precedeneresearch.com/ethanol-market
3. Wani AK, Rahayu F, Fauziah L, Suhara, C. Advances in safe processing of sugarcane and bagasse for the generation of biofuels and bioactive compounds. Journal of Agriculture and Food Research. 2023;12: Article 100549. https://www.sciencedirect.com/science/article/pii/S266615432300056X
4. Hans M, Lugani Y, Chandel AK, Rai R, Kumar S. Production of first- and second-generation ethanol for use in alcohol-based hand sanitizers and disinfectants in India. Biomass Conversion and Biorefinery. 2023;13: 7423-7440. https://doi.org/10.1007/s13399-021-01553-3
5. Cadiz JPR, Agcaoili RP, Mamuad RY, Choi AES. Fermentation of sweet sorghum (Sorghum bicolor L. Moench) using immobilized yeast (saccharomyces cerevisiae) entrapped in calcium alginate beads. Fermentation. 2023;9(3): Article 272. https://doi.org/10.3390/fermentation9030272
6. Pérez-Bermúdez I, Ribas-García M, Ibañez-Fuentes M, Saura-Laria G, Garrido-Carralero N. Análisis de la influencia de la calidad de la miel final y el tiempo perdido sobre la eficiencia industrial en la producción de etanol. ICIDCA Sobre los Derivados de la Caña de Azúcar. 2015;49(2): 58-63. https://www.redalyc.org/articulo.oa?id=223143421009
7. Amanful B, Dogbe ES, Bosman CE, Görgens JF. Stochastic techno-economic analysis for the co-production of alternative sweeteners in sugarcane biorefineries. Food and Bioproducts Processing. 2024;143: 9-20. https://www.sciencedirect.com/science/article/pii/S0960308523001220
8. Albernas Y, Corsano G, Morales M, González M, Santos R., González E. Optimal design for an etanol plant combining first and second generation Technology. Ciencia Tecnología y Futuro. 2014;5(5): 97-120. http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0122-53832014000200006
9. de Armas AC. Evaluation of second and third generation biorefinery schemes in a Cuban sugar industry. Doctoral dissertation. Universidad Central “Marta Abreu” de Las Villas, Cuba. 2019.
10. Lamas Y, de Armas AC, Albernas Y, González E. Preliminary analisys of alcoholic fermentation using filter juice, molasses and syrup mixture. Centro Azúcar. 2023;50(3): e1035. http://centroazucar.uclv.edu.cu/index.php/centro_azucar/article/view/767
11. Rojas-Sariol L, Lorenzo-Acosta Y, Domenech-López F. Estudio del consumo de ácidos en el ajuste de pH en diferentes medios de fermentación alcohólica. ICIDCA. Sobre los Derivados de la Caña de Azúcar. 2011;45(2): 57-62. https://www.redalyc.org/pdf/2231/223122259008.pdf
12. Albernas Y, González M, Corsano G, González E. Obtaining superfine ethanol in a Cuban distillery. Ingeniería e Investigación Journal. 2012;32(3): 47-52. http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-56092012000300010
13. Albernas Y, Corsano G, Kafarov V, González M, González E. Optimal design of pre-fermentation and fermentation stages applying nonlinear programming. Energy Conversion and Management. 2014;87: 1195-1201. https://www.sciencedirect.com/science/article/abs/pii/S019689041400315X
14. González M, Castellanos L, Albernas Y, González E. Process integration in a biorefinery scheme, Afinidad. 2014;71(568): 274-278. https://raco.cat/index.php/afinidad/article/view/287619
15. García R, Pérez A, Diéguez K, Mesa L, González I, González M, González E. Incorporating other substances as raw materials for fermented sugar in alcohol distilleries. Revista Facultad de Ingeniería Universidad de Antioquia. 2015;75: 130-142. https://doi.org/10.17533/udea.redin.n75a13
16. Díaz J. Valoración de alternativas para la obtención de etanol a partir de mezclas de jugos secundarios y melazas en la destilería Jesús Rabí. Master thesis, Universidad de Matanzas, Cuba. 2021. https://rein.umcc.cu/bitstream/handle/123456789/608/MSc21%20Javier.pdf?sequence=1&isAllowed=y
17. Thammasittirong SN-R, Chatwachirawong P, Khemdee, K, Thammasittirong A. Evaluating the potential of newly developed energy cane clones for first- and second-generation ethanol production. Fermentation. 2023;9(3): Article 267. https://doi.org/10.3390/fermentation9030267
18. Saura G. García R, Otero MA, Martínez JA, Bello D, Pérez I. Experiencias en la producción de etanol a partir de jugos de caña mezclados. Parte I. Materias Primas. ICIDCA sobre los derivados de la caña de azúcar. 2009;43(2): 42-46. https://www.redalyc.org/pdf/2231/223120662003.pdf
19. Rivero RP, Morales M, Mesa L. Evaluación económica de la utilización de mezclas de sustratos azucarados para la producción de etanol. Centro Azúcar. 2012;39(4): 29-35. http://centroazucar.uclv.edu.cu/index.php/centro_azucar/article/view/351
20. Martínez Y, González V, Penin E, González E. Preliminary study of the water-vinaza-flemaza mixture and its impact in the fermentation stage in the ethanol production. Tecnología Química. 2013;33(3): 206-211. http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S2224-61852013000300003
21. de la Cruz R, González E, Abreu A. Alternativas de la combinación de sustratos para la fermentación alcohólica en la destilería anexa al Complejo Agroindustrial Azucarero Melanio Hernández. Centro Azúcar. 2000;27(4): 22-28.
22. Mesa L, González E, González M, Agüero G, Benítez T. Estudio preliminar del mezclado de los sustratos: jugo de los filtros, jugos secundarios y miel en la producción de etanol. Centro Azúcar. 2006;33(4): 32-36.
23. de Armas AC, Lamas Y, Albriza MT, González E. Possibilities of utilization of filter juice in a sugar factory with distillery. El Directivo al Día. 2020;20(2): 27-36.
24. Fabelo JA. Study of the alcoholic fermentation stage using a mixture of different substrates. Doctoral dissertation. Universidad Central “Marta Abreu” de Las Villas, Cuba. 1999.
25. Morales M, Armas A, Mesa L, Acosta D, González E. Avances en el uso del licor hidrolizado de bagazo en la fermentación de mezclas azucaradas. Afinidad. 2018;75(581): 61-65. https://raco.cat/index.php/afinidad/article/view/335964
26. Mesa L, Martínez Y, de Armas AC, González E. Ethanol production from sugarcane straw using different configurations of fermentation and techno-economical evaluation of the best schemes. Renewable Energy. 2020;156: 377-388. https://doi.org/10.1016/j.renene.2020.04.091
27. Pérez A, Zumalacárregui L, Pérez O. Evaluación de tecnologías para la obtención de productos químicos de alto valor agregado y biocombustibles. Universidad y Sociedad. 2023;15(4): 138-153. https://rus.ucf.edu.cu/index.php/rus/article/view/3961
28. Guerra M, Correa Y, Medel F, Martínez L, Pérez, O. Estudio de la fermentación alcohólica con vinculación de otros sustratos. Análisis económico. Centro Azúcar. 1995;22(3): 24-30.
29. Fernández K, Morales Y, Nápoles M, González E, Cruz M. Study of the extraction of secondary juices at the Central Argentina refinery. Centro Azúcar. 2008;28(2): 43-48. http://centroazucar.uclv.edu.cu/index.php/centro_azucar/article/view/507
30. Díaz J, García A, González LY, Luis J, Díaz MLV. Adsorción de impurezas del jugo clarificado de la industria azucarera mediante biomasa pirolizada. Revista Cubana de Química. 2019;31(3): 463-477. https://cubanaquimica.uo.edu.cu/index.php/cq/article/view/5027
31. Ojeda R. Propuesta de introducción de la tecnología de obtención de alcohol orgánico en la destilería del CAI “Heriberto Duquesne”. Chemical Engineering Degree Thesis. Universidad Central “Marta Abreu” de Las Villas, Cuba. 2005. https://dspace.uclv.edu.cu/items/e8c02916-d241-4fc9-a45d-cfdfcabe317b
32. Dogbe ES, Mandegari MA, Görgens JF. Exergetic diagnosis and performance analysis of a typical sugar mill based on Aspen Plus® simulation of the process. Energy. 2018;145: 614-625. https://www.sciencedirect.com/science/article/abs/pii/S0360544217321771
33. Mensah RQ, Yingkamhaeng N, Venkatachalam P, Show P-L, Mussatto SI, Sriariyanun M., Sukyai P, Parakulsuksatid P, Rattanaporn K. Application of green produced xylooligosaccharides from sugarcane residues and their properties – Recent progress towards sustainability. Bioresource Technology Reports, 2023;23: Article 101537. https://www.sciencedirect.com/science/article/abs/pii/S2589014X23002086
34. Carvalho LC, Oliveira ALS, Carsanba E, Pintado M, Oliveira C, Phenolic compounds modulation in β-farnesene fed-batch fermentation using sugarcane syrup as feedstock. Industrial Crops and Products. 2022;188(B): Article 115721. https://www.sciencedirect.com/science/article/abs/pii/S0926669022012043
35. de Armas AC, Morales M, Albernas Y, González E. Projection of a sugar industry to become a biorefinery from second and third generation biofuels. Tecnología Química. 2019;39(3): pp. 489-507. http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S2224-61852019000300489
36. NC 290: 2007. Norma Cubana Bebidas alcohólicas-Determinación del grado alcohólico en alcoholes, bebidas alcohólicas destiladas, vinos, licores, bebidas alcohólicas preparadas, cocteles y extractos hidroalcohólicos. Oficina Nacional de Normalización. 2007. Available from: https://ftp.isdi.co.cu/Biblioteca/BIBLIOTECA%20UNIVERSITARIA%20DEL%20ISDI/COLECCION%20DIGITAL%20DE%20NORMAS%20CUBANAS/2007/NC%20290%20%20a2007%20%20324p%20twi.pdf
37. NC 709: 2009. Norma Cubana MIEL FINAL - Determinación de sólidos aerométricos disueltos. Oficina Nacional de Normalización. 2009. Available from: https://ftp.isdi.co.cu/Biblioteca/BIBLIOTECA%20UNIVERSITARIA%20DEL%20ISDI/COLECCION%20DIGITAL%20DE%20NORMAS%20CUBANAS/2013/NC%20710%20a2013%2014p%20isp.pdf
38. NC 711: 2009. Determinación potenciométrica del pH. Oficina Nacional de Normalización. 2009. Available from: https://ftp.isdi.co.cu/Biblioteca/BIBLIOTECA%20UNIVERSITARIA%20DEL%20ISDI/COLECCION%20DIGITAL%20DE%20NORMAS%20CUBANAS/2009/NC%20711%20%20a2009%205p%20kal.pdf
39. NC 790: 2010. Bebidas alcohólicas - Determinación del grado alcohólico - métodos de referencia: 1- grado alcohólico por picnometría y 2- grado alcohólico por densimetría dígital. Oficina Nacional de Normalización. 2010. Available from: https://ftp.isdi.co.cu/Biblioteca/BIBLIOTECA%20UNIVERSITARIA%20DEL%20ISDI/COLECCION%20DIGITAL%20DE%20NORMAS%20CUBANAS/2010/NC%20790%20a2010%2011p%20qjc.pdf
40. Cortés MF, de Armas AC, Alomá IC, Morales M. Filter juice extraction impact on an industrial sugar complex sustainability. Centro Azúcar. 2021;48(1): 59-70. http://centroazucar.uclv.edu.cu/index.php/centro_azucar/article/view/642
41. de Armas AC, Albernas Y, Lamas Y, Benítez R, Dueñas A. Fermentación alcohólica de mezclas de jugo de los filtros, miel final y meladura: Estudio preliminar en una destilería cubana. Proceedings of the VI Congreso Internacional de Ingeniería Aplicada in VII Convención Científica Internacional UTM 2023. Portoviejo (Manabí, Ecuador); 2023 Oct 24-27.
42. Sablayrolles J-M. Kinetics and control of alcoholic fermentation during wine production. Chapter of: Yeasts in the production of wine, Springer New York, NY; 2019. p. 283-313. https://doi.org/10.1007/978-1-4939-9782-4_9
43. Ribeiro MLD, Ferreira OE, Teixeira V, Mutton MA, Mutton MJR. Physicochemical treatment of sugarcane juice produces quality cachaça. Agronomic Science Magazine. 2017;48(3): 458-463. https://doi.org/10.5935/1806-6690.20170053
Received: September 23, 2024 / Accepted: November 3, 2024 / Published: December 15, 2024
Citation: Albernas Y, Lamas Y, de Armas AC, Gallardo I. Approach before alcoholic fermentation of mixtures with syrup in a Cuban distillery. Bionatura journal. 2024;1(4):17. doi: 10.70099/BJ/2024.01.04.17
Additional information Correspondence should be addressed to yailetac@uclv.edu.cu
Peer review information. Bionatura thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
ISSN.3020-7886
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Publisher's Note: Bionatura Journal stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2024 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


