Candida auris: An Emerging Challenge in Clinical Mycology and Public Health in Latin America
Jaime David Acosta-España 1,2,3,4,5*,, Alexander Maldonado 1,4 , Andrés Herrera-Yela 1,4,*
1 Facultad de Ciencias de la Salud, Universidad Internacional SEK del Ecuador
; jaime.acosta@uisek.edu.ec .
2 Escuela de medicina, Pontificia Universidad Católica del Ecuador; jdacosta@puce.edu.ec.
3 Instituto de Microbiología, Universidad Friedrich Schiller de Jena, Jena, Alemania; jaime.david.acosta.espana@leibniz-hki.de
4 Grupo de Investigación de Enfermedades Emergentes y Desatendidas, Ecoepidemiología y Biodiversidad, Facultad de Ciencias de la Salud, Universidad Internacional SEK (UISEK), Quito 170120, Ecuador; ruben.maldonado@uisek.edu.ec, manuel.herrera@uisek.edu.ec.
5 Centro de Investigación para la Salud en América Latina (CISeAL), Pontificia Universidad Católica del Ecuador, Quito, Ecuador
* Correspondence: jaime.acosta@uisek.edu.ec, jdacosta@puce.edu.ec
ABSTRACT
Candida auris is an emerging fungus that poses a critical challenge in clinical mycology and global public health. Identified in 2005, this pathogen has caused hospital outbreaks due to its resistance to multiple antifungals and ability to persist in hospital settings. In Latin America, C. auris has been reported since 2012 in several countries, including a case in Ecuador in 2024. Its emergence has been linked to climate change and the excessive use of antifungals, which could be favoring its proliferation. The diagnosis of C. auris is complex and requires advanced methods such as MALDI-TOF mass spectrometry and molecular techniques due to its phenotypic variability. Another factor to be relevant to public health is resistance to conventional treatments. In addition, genomic surveillance is essential to understand the epidemiology of this pathogen and control its spread in hospital settings. Urgent prevention, detection, and treatment measures are necessary to limit the spread of C. auris in the region and minimize its impact on public health. Implementing comprehensive strategies, including genomic surveillance and advanced diagnostic techniques, is crucial to address this emerging problem in Latin America.
Keywords: Candida auris, Fungal infections, Antifungal resistance, Clinical mycology, Genomic surveillance, Latin America.
INTRODUCTION
Candida auris is an emerging fungus that has garnered global attention due to its significant impact on clinical mycology and public health. First identified in Japan in 20051 , this pathogen has been responsible for numerous hospital outbreaks worldwide, notable for its resistance to multiple antifungal agents2–4 . Its ability to colonize hospital environments and cause severe infections in vulnerable patients has raised serious concerns within the medical community. The high mortality rate associated with these infections, combined with the challenges in diagnosis and treatment, underscores the need for rigorous epidemiological surveillance and effective control strategies 2,3,5,6 . In Latin America, the rapid and concerning spread of C. auris highlights the importance of addressing this issue from a regional and global perspective 7,8 . This editorial explores the epidemiology, risk factors, clinical presentation, diagnosis, treatment, genomic surveillance, and phylogeny of C. auris, emphasizing the urgent need for comprehensive measures to manage it in the region.
Candida auris is an emerging fungus that has garnered global attention due to its significant impact on clinical mycology and public health. First identified in Japan in 2005
Phylogeny and Global Distribution of Candida auris
Candida auris has emerged as a globally concerning pathogen, exhibiting a complex phylogeny that highlights its remarkable ability to adapt and spread in diverse environments. Phylogenetically, C. auris is closely related to species like Candida haemulonii, yet it is distinctly characterized by significant genetic variations that justify its classification as a separate species (Table 1A)9,10 .
Four main lineages of C. auris have been identified, each associated with a distinct geographic region: the South Asian clade, the East Asian clade, the African clade, and the South American clade10,11 . These lineages demonstrate the genetic diversity within the species and reflect geographic dispersal patterns that may influence both virulence and antifungal resistance. Additionally, recent studies have identified new lineages linked to regions such as Iran (fifth clade) and Singapore/Bangladesh (sixth clade), suggesting greater complexity in the genetic structure of this species 10 .
The global spread of C. auris is alarming, with reports of cases in over 40 countries since its identification in Japan in 2005. Its presence across multiple continents, including outbreaks in Venezuela, Colombia, India, South Africa, and more recently Ecuador, underscores its ability to persist in hospital environments and resist conventional antifungal treatments. Its nosocomial transmission and ability to colonize inert surfaces facilitate this widespread distribution. Understanding the phylogeny and global distribution of C. auris is crucial for developing effective control strategies. Different lineages may exhibit variations in antifungal resistance, making it essential to tailor therapeutic and preventive interventions according to the pathogen's genetic diversity6,7,10 .
Candida auris has emerged as a globally concerning pathogen, exhibiting a complex phylogeny that highlights its remarkable ability to adapt and spread in diverse environments. Phylogenetically, C. auris is closely related to species like Candida haemulonii, yet it is distinctly characterized by significant genetic variations that justify its classification as a separate species (Table 1A)
Four main lineages of C. auris have been identified, each associated with a distinct geographic region: the South Asian clade, the East Asian clade, the African clade, and the South American clade
The global spread of C. auris is alarming, with reports of cases in over 40 countries since its identification in Japan in 2005. Its presence across multiple continents, including outbreaks in Venezuela, Colombia, India, South Africa, and more recently Ecuador, underscores its ability to persist in hospital environments and resist conventional antifungal treatments. Its nosocomial transmission and ability to colonize inert surfaces facilitate this widespread distribution. Understanding the phylogeny and global distribution of C. auris is crucial for developing effective control strategies. Different lineages may exhibit variations in antifungal resistance, making it essential to tailor therapeutic and preventive interventions according to the pathogen's genetic diversity
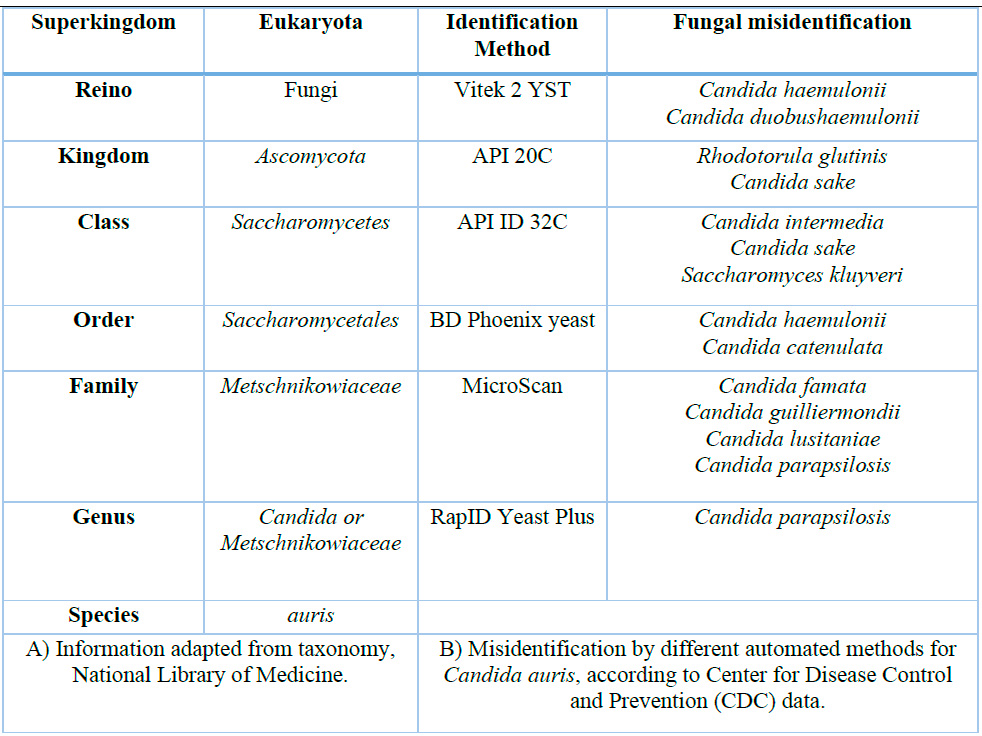
A) Information adapted from taxonomy, National Library of Medicine.
B) Misidentification by different automated methods for Candida auris, according to Center for Disease Control and Prevention (CDC) data.
Table 1.- Candida auris taxonomy and automated or semi-automated microbiological identification methods.
Epidemiology of Candida auris in Latin America
Since its first outbreak in Venezuela in 2012, Candida auris has rapidly spread across Latin America. Countries such as Colombia, the United States, Panama, Canada, Chile, and Costa Rica have reported subsequent cases, with a recent outbreak in Ecuador in February 2024, according to an unpublished epidemiological bulletin7,8 . This fungus is known for its ability to persist on inanimate surfaces and resist disinfection, facilitating its transmission in hospitals. Its resistance to multiple antifungal agents complicates outbreak control and increases associated mortality. Furthermore, limited infrastructure and resource constraints in some countries hinder the implementation of effective surveillance and control measures. The rapid dissemination of C. auris in the region highlights the need for a coordinated response that includes epidemiological surveillance, healthcare worker training, and access to advanced diagnostic tools to prevent and control the spread of this emerging pathogen 8 .
Since its first outbreak in Venezuela in 2012, Candida auris has rapidly spread across Latin America. Countries such as Colombia, the United States, Panama, Canada, Chile, and Costa Rica have reported subsequent cases, with a recent outbreak in Ecuador in February 2024, according to an unpublished epidemiological bulletin
Climate Change and the Emergence of Candida auris
Climate change has been identified as a critical factor in the emergence of Candida auris7 . Changes in climate, such as rising temperatures, may have created more favorable conditions for the proliferation of C. auris. This fungus has shown a remarkable ability to survive at elevated temperatures, suggesting a possible thermal adaptation linked to climate change. Furthermore, the extensive use of antifungals in agriculture and medicine has exerted selective pressure on various fungal species, promoting the emergence of resistant strains. C. auris has demonstrated resistance to multiple antifungals and an ability to persist in hospital environments, increasing its potential for nosocomial transmission. These dynamics highlight the need to consider climate change as a contributing factor in the evolution and emergence of fungal pathogens and underscore the urgency of integrating human, animal, and environmental health into mitigation strategies 12,13 .
Climate change has been identified as a critical factor in the emergence of Candida auris
Infection Risk Factors
Key risk factors include prior colonization, prolonged stays in intensive care units, and invasive medical devices such as catheters and ventilators4,6,13 . Immunosuppression, recent surgeries, and previous exposure to antibiotics and antifungals also significantly increase the risk of infection (Figure 1A). Patients in intensive care, especially those with multiple comorbidities, are particularly vulnerable. The ability of C. auris to persist on surfaces and medical devices facilitates its spread in hospital environments, making infections caused by this fungus a significant challenge in managing healthcare-associated infections (HAIs). Identifying and managing these risk factors is crucial to reducing the incidence of C. auris infections and improving hospital preventive measures 2,14 .
Key risk factors include prior colonization, prolonged stays in intensive care units, and invasive medical devices such as catheters and ventilators
Clinical Presentation
Infections caused by Candida auris present with nonspecific clinical symptoms, complicating both diagnosis and treatment2,9,13–15 . Patients typically experience fever and chills that do not respond to conventional antimicrobial therapy (Figure 1B), potentially delaying appropriate diagnosis 2,14 . C. auris can cause severe invasive infections in the bloodstream, wounds, and other organs and is particularly dangerous due to its high mortality rate 2 . Its ability to form biofilms on medical devices such as catheters and ventilators contributes to its persistence and resistance to treatment 13 . Since C. auris infections can mimic other fungal infections, it is crucial to consider this pathogen in patients not responding to antibiotic and antifungal treatments.
Infections caused by Candida auris present with nonspecific clinical symptoms, complicating both diagnosis and treatment
Diagnosis and Treatment of Candida auris
Diagnosing Candida auris is complex due to its tendency to be misidentified as other Candida species using conventional methods (Table 1B)5,9 . Advanced techniques such as MALDI-TOF mass spectrometry and Polymerase Chain Reaction (PCR) are essential for accurate identification (Figure 1C). These tools facilitate faster diagnosis and are crucial for guiding appropriate treatment. C. auris is resistant to multiple classes of antifungals, making treatment challenging 3,9,14 . An antifungal susceptibility test is critical to determine the fungus's resistance profile and guide treatment choices 2,3 . Resistance to azoles, echinocandins, and amphotericin B is standard, limiting therapeutic options (Figure 1D). However, the drug ibrexafungerp has shown in vitro efficacy against C. auris, offering a promising new treatment option 3,4,13–15 . Ongoing research is necessary to develop new therapies and address the growing antifungal resistance.
Diagnosing Candida auris is complex due to its tendency to be misidentified as other Candida species using conventional methods (Table 1B)
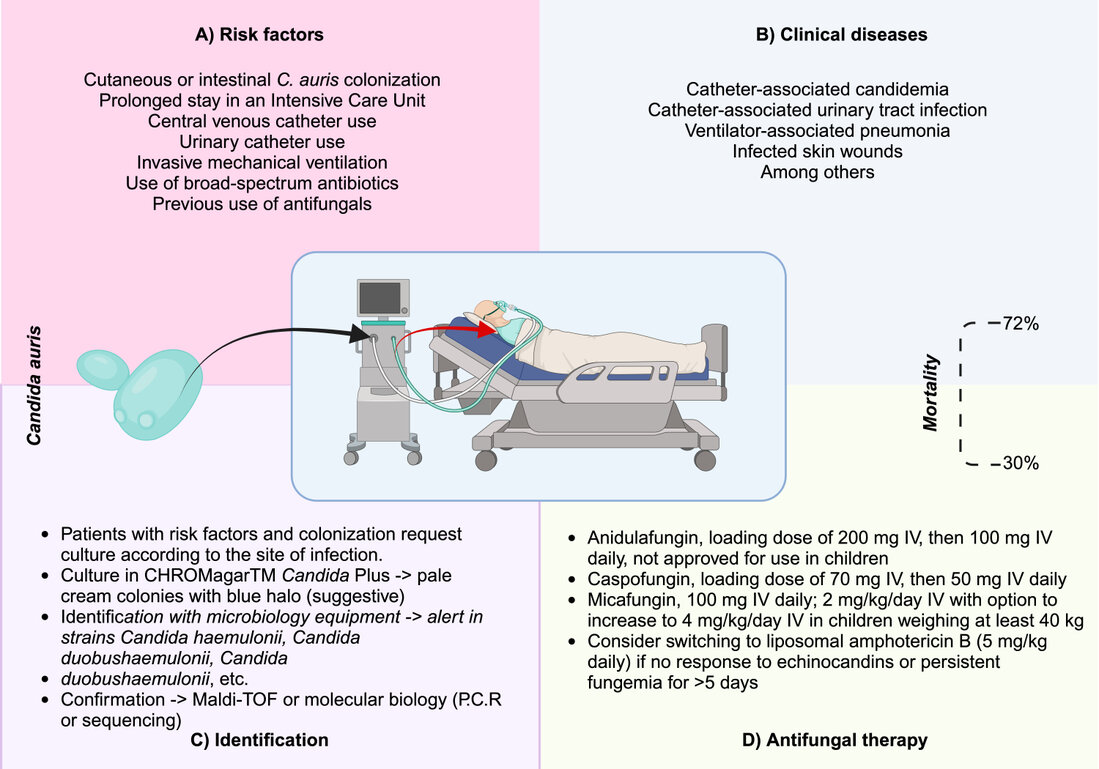
Figure 1. Evidence-based depiction of Candida auris infection. A) Risk factors for C. auris infection. B) Most common clinical diseases. C) Laboratory identification recommendations. D) CDC-recommended therapy. The budding yeast of C. auris is green and can colonize hospital environments and medical devices such as ventilators, central venous catheters, and urinary catheters. The red arrow indicates transmission from these devices to the patient with risk factors, facilitating invasion and infection—image created in Biorender.
Importance of Genomic Surveillance
Genomic surveillance is a vital tool in controlling Candida auris. Whole-genome sequencing allows for the identification of genetic variants and antifungal resistance genes, which is crucial for understanding the epidemiology of C. auris and its capacity to spread 16. It also aids in identifying specific lineages and tracking outbreaks in hospital settings, enabling a rapid and effective response to new cases and helping to mitigate its impact on global health16 .
Currently, the NCBI (National Center for Biotechnology Information) database contains 212 complete C. auris genomes from 29 countries (Figure 2), emphasizing the importance of global genomic surveillance. This approach allows for a detailed analysis of the pathogen's epidemiology and more effective therapeutic strategies based on the early identification of antifungal resistance genes 16, 17.
Integrating genomic surveillance into C. auris diagnostic and treatment protocols are critical for strengthening control measures and improving available therapies. By leveraging the growing accessibility of next-generation sequencing technologies, public health systems can anticipate the pathogen's evolution and reduce its impact on human health, especially in increasing antifungal resistance. Therefore, genomic surveillance represents an advanced and comprehensive strategy for preventing and treating infections caused by this pathogen 16, 17.
Genomic surveillance is a vital tool in controlling Candida auris. Whole-genome sequencing allows for the identification of genetic variants and antifungal resistance genes, which is crucial for understanding the epidemiology of C. auris and its capacity to spread 16. It also aids in identifying specific lineages and tracking outbreaks in hospital settings, enabling a rapid and effective response to new cases and helping to mitigate its impact on global health
Currently, the NCBI (National Center for Biotechnology Information) database contains 212 complete C. auris genomes from 29 countries (Figure 2), emphasizing the importance of global genomic surveillance. This approach allows for a detailed analysis of the pathogen's epidemiology and more effective therapeutic strategies based on the early identification of antifungal resistance genes 16, 17.
Integrating genomic surveillance into C. auris diagnostic and treatment protocols are critical for strengthening control measures and improving available therapies. By leveraging the growing accessibility of next-generation sequencing technologies, public health systems can anticipate the pathogen's evolution and reduce its impact on human health, especially in increasing antifungal resistance. Therefore, genomic surveillance represents an advanced and comprehensive strategy for preventing and treating infections caused by this pathogen 16, 17.
Distribution by Country of 212 Complete Candida auris Genomes Uploaded to the NCBI Database (National Center for Biotechnology Information). Update Date: September 4, 2024.

Figure 2. Heatmap of Complete Candida auris Genomes in NCBI by Country.
CONCLUSIONS
The spread of Candida auris in Latin America represents a critical challenge for public health and clinical mycology. Its resistance to multiple antifungals and its ability to persist in hospital environments and cause severe infections underscores the need for a comprehensive response that includes epidemiological surveillance, advanced diagnostics, appropriate treatment, and genomic monitoring. Climate change and the overuse of antifungals have contributed to the emergence of this pathogen, reinforcing the need to consider these factors in mitigation strategies. Accurate identification of C. auris, combined with understanding its phylogeny and implementing new therapies, is crucial to controlling its spread and reducing its impact on human health. Collaboration between health systems and ongoing research is essential to address this emerging challenge and protect public health in the region.
Supplementary Materials: None.
Author Contributions: JDA-E conceptualized the original idea, conducted the synthesis of information, drafted the initial manuscript, and reviewed and approved all versions. AM contributed to the initial drafting of the manuscript and reviewed and approved all versions. AH-Y evaluated Candida auris genomes in the NCBI database, created the heatmap, included perspectives on genomic surveillance, and reviewed and approved all manuscript versions.
Funding: None.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: No applicable
Data Availability Statement: The relevant information is included in the manuscript.
Acknowledgments: We thank the institutions affiliated with the authors for their unconditional support of this research.
Conflicts of Interest: None to declare.
REFERENCES
1. Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol [homepage on the Internet] 2009 [cited 2024 Mar 20];53(1):41–44. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1348-0421.2008.00083.x
2. Cristina ML, Spagnolo AM, Sartini M, et al. An Overview on Candida auris in Healthcare Settings. Journal of Fungi 2023, Vol 9, Page 913 [homepage on the Internet] 2023 [cited 2024 Mar 20];9(9):913. Available from: https://www.mdpi.com/2309-608X/9/9/913/htm
3. Ademe M, Girma F. Candida auris: From Multidrug Resistance to Pan-Resistant Strains. Infect Drug Resist [homepage on the Internet] 2020 [cited 2024 Mar 20];13:1287. Available from: /pmc/articles/PMC7211321/
4. Organización Panamericana de la Salud. Candida auris outbreaks in health care services in the context of the COVID-19 pandemic [Homepage on the Internet]. Washintong D.C: 2021 [cited 2024 Mar 20]; Available from: https://iris.paho.org/bitstream/handle/10665.2/53377/EpiUpdate6February2021_eng.pdf?sequence=1&isAllowed=y
5. Leonhard SE, Chong GM, Foudraine DE, et al. Proposal for a screening protocol for Candida auris colonization. Journal of Hospital Infection [homepage on the Internet] 2024 [cited 2024 Mar 25];146:31–36. Available from: http://www.journalofhospitalinfection.com/article/S0195670124000306/fulltext
6. Alvarez-Moreno CA, Morales-López S, Rodriguez GJ, et al. The Mortality Attributable to Candidemia in C. auris Is Higher than That in Other Candida Species: Myth or Reality? Journal of Fungi 2023, Vol 9, Page 430 [homepage on the Internet] 2023 [cited 2024 Mar 20];9(4):430. Available from: https://www.mdpi.com/2309-608X/9/4/430/htm
7. Ellwanger JH, Chies JAB. Candida auris emergence as a consequence of climate change: Impacts on Americas and the need to contain greenhouse gas emissions. The Lancet Regional Health - Americas [homepage on the Internet] 2022 [cited 2024 Mar 20];11:100250. Available from: http://www.thelancet.com/article/S2667193X22000679/fulltext
8. Pan American Health Organization. Epidemiological Alert: Candida auris outbreaks in health care services in the context of the COVID-19 pandemic - 6 February 2021 - PAHO/WHO | Pan American Health Organization [Homepage on the Internet]. World Health Organization. 2021 [cited 2021 Feb 21];Available from: https://www.paho.org/en/documents/epidemiological-alert-candida-auris-outbreaks-health-care-services-context-covid-19
9. Identification of Candida auris | Candida auris | Fungal Diseases | CDC [Homepage on the Internet]. [cited 2024 Mar 20];Available from: https://www.cdc.gov/fungal/candida-auris/identification.html
10. Chow NA, Muñoz JF, Gade L, et al. Tracing the Evolutionary History and Global Expansion of Candida auris Using Population Genomic Analyses. mBio 2020;11(2).
11. Santana DJ, Zhao G, O'Meara TR. The many faces of Candida auris: Phenotypic and strain variation in an emerging pathogen. PLoS Pathog [homepage on the Internet] 2024 [cited 2024 Mar 25];20(3):e1012011. Available from: https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1012011
12. Hofer U. Candida auris' potential link to climate change. Nat Rev Microbiol 2019;17(10):588–588.
13. Frías-De-León MG, García-Salazar E, Reyes-Montes M del R, Duarte-Escalante E, Acosta-Altamirano G. Opportunistic Yeast Infections and Climate Change: The Emergence of Candida auris. 2022 [cited 2023 Feb 2];161–179. Available from: https://link.springer.com/chapter/10.1007/978-3-030-89664-5_10
14. National Library of Medicine. Candida auris [Homepage on the Internet]. 2024 [cited 2024 Mar 19];Available from: https://www.ncbi.nlm.nih.gov/datasets/taxonomy/498019/
15. Lee WG, Shin JH, Uh Y, et al. First Three Reported Cases of Nosocomial Fungemia Caused by Candida auris. J Clin Microbiol [homepage on the Internet] 2011 [cited 2024 Mar 20];49(9):3139. Available from: /pmc/articles/PMC3165631/
16. Werner G, Couto N, Feil EJ, et al. Taking hospital pathogen surveillance to the next level. Microb Genom 2023;9(4).
Received: August 21, 2024 / Accepted: 1 September, 2024 / Published: 15 September 2024
Citation: Acosta-España J, Maldonado A, Herrera-Yela A. Candida auris: An Emerging Challenge in Clini-cal Mycology and Public Health in Latin America. Bionatura journal. 2024;1(3):23. doi: 10.70099/BJ/2024.01.03.23
Additional information Correspondence should be addressed to jaime.acosta@uisek.edu.ec, jdacosta@puce.edu.ec
Peer review information. Bionatura thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
ISSN.3020-7886
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Publisher's Note: Bionatura Journal stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2024 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


