Innovative protection: characterization of edible cassava and lavender coatings for sustainable packaging solutions
Irvin Tubon 1*, Goering Octavio Zambrano-Cárdenas 2, Jessica Paola Arcos-Logroño3,
Cristian Germán Santiana-Espín 4, Gabriela Liseth Vaca-Altamirano 5,
1 Escuela Superior Politécnica de Chimborazo, Grupo de Investigación en Biomedicina, Tecnología y Atención Farmacéutica de Ecuador, Carrera de Medicina, Facultad de Salud Pública, Riobamba - Ecuador; itubon@espoch.edu.ec..
2 Escuela Superior Politécnica de Chimborazo, Sede Morona Santiago, Carrera de Ingeniera Ambiental, Macas – Ecuador; goering.zambrano@espoch.edu.ec ..
3 Escuela Superior Politécnica de Chimborazo, Sede Morona Santiago, Carrera de Ingeniera Ambiental, Macas – Ecuador; paola.arcos@espoch.edu.ec..
4 Escuela Superior Politécnica de Chimborazo. Carrera de Agroindustria, Facultad de Ciencias Pecuarias, Riobamba – Ecuador; cristian.santiana@espoch.edu.ec..
5 Universidad Regional Autónoma de los Andes, Facultad de Ciencias Médicas, Carrera de Odontología. Ambato. Ecuador; ua.gabrielavaca@uniandes.edu.ec..
* Correspondence: itubon@espoch.edu.ec.
ABSTRACT
Developing edible coatings using biopolymers and natural bioactive compounds has recently received increased attention. These coatings present an environmentally friendly packaging solution that is recognized for its sustainability and consumer appeal, unlike traditional methods of food preservation. Thus, the main objective of this research was to develop coatings from Manihot esculenta Crantz containing different concentrations of Lavandula angustifolia Mill essential oil. Therefore, the influence of varying the concentration of the essential oil on the physical and chemical characteristics, as well as on the antimicrobial efficacy, was evaluated. The findings revealed a remarkable impact of the essential oil on the coating, resulting in an increase in thickness and a decrease in solubility, swelling capacity, water vapor transmission rate, and water vapor permeability. It is essential to highlight the potential of the developed coatings to inhibit the growth of common pathogenic bacteria, indicating their feasibility in protecting foods from bacterial spoilage and prolonging their shelf life.
Keywords: Cassava starch; lavender; coating; essential oil; innovation.
INTRODUCTION
Packaging extends the shelf life of different products, protecting them from mechanical, chemical, and biological contamination 1 . However, plastic packaging is one of the primary solid wastes in most cities worldwide, and much of it is not degradable in a typical environment 2,3 .
Different new food packaging concepts have been introduced to meet consumer demands. Thus, innovative materials like active packaging have been developed to prolong storage and maintain product quality 4.
Edible coatings usually include different hydrophilic components, such as polysaccharides or proteins. These natural biopolymers can provide mechanical protection, act as barriers to mass transfers (such as gases and water vapor), and even carry functional additives (such as antimicrobial agents and antioxidants) to extend the shelf life of food products3 effectively5 . Thus, biopolymers are now an alternative in the food industry 4–6 .
Regarding tubers, their main component, starch, can produce biodegradable films on a large scale and cheaply. Therefore, starch-based materials can reduce disposable plastic and promote alternative packaging to reduce environmental pollution 7,8 .
Since the last 30 years, cassava production has almost doubled to about 260 million tons in 2012, making it an abundant and attractive source for new research. The most significant production is in Africa, one-third in Asia, and 14% in Latin America. Thus, cassava starch has become one of the most promising raw materials for producing edible coatings and films due to its low cost, abundance, renewability, biodegradability, and film-forming capacity 9,10 .
Food safety is a global health concern, which has generated considerable attention to the presence of foodborne pathogens due to the risk to health and the increasing development of antibiotic resistance 11 .
On the other hand, essential oils consist of the lipophilic fraction of the phytocomplex, which includes terpenes and some phenolic compounds. They are synthesized in specific glandular tissues to attract animals for pollen and seed dispersal and to protect plants from abiotic and biotic environmental stressors 12 . Nevertheless, in plants, the primary function of essential oils is represented by their inhibitory effect against bacteria, fungi, and viruses 13
One of them, lavender (Lavandula angustifolia Mill.), is a species of the Lamiaceae family used to relieve mild symptoms of mental stress, exhaustion and the treatment of insomnia 14 . For this reason, today, oil and its products are among the most popular worldwide. In addition, recent studies have described its antibacterial and anticarcinogenic effect 15 .
Based on this, the present research determined the antimicrobial capacity of an edible coating made from cassava starch as a possible alternative to protect and preserve food.
MATERIAL AND METHODS
Raw material procurement
No specific methodology was used to prepare the materials purchased from local companies "Las Esencias y Supermaxi." The materials included analytical-grade starch, gelatin, glycerol, essential oil, amylose, and amylopectin stocks. Inhibition disks gentamicin (30 μg/disk), chloramphenicol (30 μg/disk), and tetracycline (30 μg/disk) were obtained from the company "Karbasth Distribuciones." The lavender essential oil obtained from the manufacturer had the following composition: linalool (31,77%), lilanyl acetate (28,60%), camphor (8,30%), and 1.8 cineole (6,57%).
Amylose and Amylopectin Determination
The concentration of amylose and amylopectin present in cassava starch was determined following the guidelines established in ISO 6647-1:2020 16 . For this, 100 mg of the sample (cassava starch) was taken, and 1 mL of ethanol and 9 mL of 1 M sodium hydroxide solution were added. The mixture was gently stirred and placed in a water bath (GP05, THERMO SCIENTIFIC) for 10 min. The blank solution and various stocks of amylose and amylopectin were prepared similarly. Then, 5 mL of each prepared solution was pipetted into flasks containing 50 mL of distilled water, and 1 mL of acetic acid was added. Subsequently, 2 mL of saturated iodine solution was added, mixed, and allowed to stand for 10 minutes. The absorbance was measured at 620 nm against the blank solution using a Multiskan FC microplate spectrophotometer (Thermo Scientific). The percentages of amylose and amylopectin were obtained by plotting the amylose and amylopectin calibration curves at various concentrations.
Preparation of the coating
The biodegradable coating was prepared using the casting method. To prepare the coating, 5% w/v cassava starch, 3% w/v glycerol, 5% w/v gelatin, and varying amounts of distilled water were mixed, depending on the concentration of essential oil (EO) used. The mixture was heated to 96 °C for 40 minutes while constantly stirring to ensure the starch was gelatinized. After cooling to approximately 40 °C, the EO was immediately added at concentrations of 1%, 2,5%, 5%, and 10%. The gel was then degassed by applying vacuum for 7 minutes and poured into polypropylene plates, which were dried in an incubator (IN30, Memmet) at 45 °C for 48 hours 18 .
Thickness
The thickness was measured using a micrometer (Rexbeti RXD-0), for which five different points were randomly selected from each cover. The values were recorded, and an average was obtained 19 . This procedure was performed in triplicate on each coating.
Moisture content (MC)
The moisture content of the biodegradable cover was determined by taking a sample of the cover (20 mm x 20 mm) and weighing it on an analytical balance (AS 120, RADWAG). The weight (M0) was noted, and the sample was then dried in a convection oven (AR-290, ARSA) at 110°C for 24 hours until a constant weight was obtained (M1). This process was repeated three times for each coating formulation, and the values were recorded to obtain an average. Moisture content was calculated using the following equation (1) 20 :

Solubility
Samples of each coating (20 mm × 20 mm) were dried in a convection oven (AR-290, ARSA) at 105°C for 24 hours and weighed to obtain the initial weight (M0). The samples were then immersed in 50 mL of distilled water in a sealed beaker to prevent evaporation and dust ingress. They were kept at 25°C for 24 hours with periodic stirring at 30 rpm. Afterward, the remaining coverslips were placed on Whatman filter paper (grade 201) to remove the remaining water. Finally, they were dried at 80°C until a constant weight (M1) was obtained. The tests were performed in triplicate, and solubility values were calculated for each sample as follows (2) 21 :

Swelling Capacity (SC)
Samples were collected from each coating (40 mm × 20 mm), dried at 60°C for 24 hours, weighed (M0), and dipped in a 50 mL beaker containing 0,1 M NaCl solution. The beaker was sealed to avoid dust and prevent evaporation. After 30 minutes, the samples were removed from the solution, and excess water was removed between two filter papers. The samples were then weighed again (M1). This process was repeated three times for each coating. The swelling capacity value was calculated as follows (3) 22 :

Water Vapor Permeability (WVP)
The coating samples' water vapor permeability (WVP) was determined using gravimetry following the ASTM E95-E96 standard method with some modifications. To do this, beakers were filled with 10 g of silica gel (0% relative humidity), and each conditioned coating sample was placed on top. The beakers were then placed in a vessel containing a saturated NaCl solution (40 g NaCl in 100 g H2O) (75% relative humidity), and the system was kept at 25°C. The weight of the samples was measured every hour for 8 hours. This process was carried out three times per coating. The WVP was calculated using the given equation (4) 22,23 :

WVTR: water vapor transmission, corresponding to the mass change curve's slope as a time function (g/h).
: average thickness of the coating (mm).
P0: saturation vapor pressure at 25° C
RH1 – RH2: difference in relative humidity of silica gel (0%) and NaCl solution (0,75%).
Antibacterial activity
The antimicrobial activity of the coating formulations was determined using the disk diffusion method. In this method, 0,1 mL of the inoculum, which was equivalent to 0,5 on the McFarland scale (1,5 x 108 cells/mL), of each strain (Escherichia coli ATCC 11229 and Staphylococcus aureus ATCC 13150) was seeded on Mueller-Hinton agar plates 24 .
From each prepared coating, 6 mm diameter discs were cut and placed on the agar surface with sterile forceps (four discs on each plate). Antibiotic discs of gentamicin (30 μg/disc), chloramphenicol (30 μg/disc), and tetracycline (30 μg/disc) were used as positive controls. Filter paper discs containing sterile water were used as negative controls. The plates were then incubated at 37 ± 0,5 ºC for 24 h. After incubation, the diameter of the area around the disk (halo), expressed in millimeters, including the disk diameter, was measured. Tests were performed in triplicate for each coating formulation 23,24 .
The sensitivity of Escherichia coli ATCC 11229 pathogens and Staphylococcus aureus ATCC 13150 against the coatings were classified according to the diameter of the inhibition halos. The classification was based on Weintein et al., 2020 25, and is as follows: not sensitive (-) for diameters less than 8 mm; sensitive (+) for diameters of 9-14 mm; very sensitive (++) for diameters of 15 to 19 mm; extremely sensitive (+++), for diameters more significant than 20 mm.
Statistical Analysis
The data were processed using STATGRAPHICS statistical software. The results were analyzed using one-way analysis of variance (ANOVA) and compared using the Tukey multiple comparisons test at a 95% confidence level (α = 0,05).
RESULTS AND DISCUSSION
Coating Films
Yuca starch is a polymer that can produce odorless and colorless biodegradable films, visually comparable to traditional plastics 26 . Several types of research demonstrate the feasibility of using yuca starch as the main biopolymer for manufacturing biodegradable films and coatings 27-31 .
Amylose in yuca starch plays a vital role in the retrograde process since the degree of polymerization depends directly on the percentage of amylose 32 . The yuca starch used in this study contained 16,54% starch, which is comparable to studies such as Aristizábal & Sánchez, (2007) 26 and He et al. (2020) 33 , in which an average of 17% starch was obtained. This feature makes yuca starch an ideal candidate for producing biodegradable films due to the ability of starch to create stable hydrogen bonds between the polymer chains.
Furthermore, Mohamed et al. (2020) 34 present various formulations studied for preparing coating, highlighting those formulated based on polysaccharides/proteins. Gelatin was chosen as a coadjuvant as it improves the mechanical characteristics of the coatings and is low-cost and easy-to-acquire reactive 22 . According to Al-Hassan & Norziah, (2012) 35, a concentration (1:1) should be chosen between gelatin and starch because a highly stable polymeric matrix is generated, in which the hydroxyl groups (-OH) of amylose interact with the amide groups (-NH) of proteins, forming molecular solid interactions. However, Tongdeesoontorn et al. (2012) 21 show that gelatine can increase the water vapor permeability of the coating.
On the other hand, lavender essential oil is widely used in industry because it has several functional volatile components, which gives it a sizeable inhibitory spectrum. Adding lavender essential oil to the formulation gives the covered barriers antimicrobial protection; in turn, the oil's hydrophobic character helps reduce the polymer matrix's molecular interactions with water 36,37
Table 1 shows a progression of color change and appearance of the cover as the concentration of the essential oil increases. Previous research, such as Medina et al. (2015) 31 and Šuput et al. (2016) 38, show that embedded oils affect the visual characteristics of coatings because the essential oils have a characteristic color and smell.

Table 1. Coatings based on cassava starch and gelatin incorporated with different concentrations of lavender (Lavandula Angustifolia Mill) essential oil are presented. R: number of replicates.
In addition, the gradual incorporation of the essential oil contributes to irregularities on the surface of the film compared to the control; this is illustrated by Supardan et al. (2016) 39, who, making use of AFM (Atomic Force Microscopy), demonstrate that the surface is rougher and more irregular in the films with the higher concentrations of the essential oil.
Thickness
The edible coatings developed in this research exhibit an average thickness between 144 ± 13,12 μm to 498 ± 23,58 μm depending on the concentration of the essential oil. Figure 1 shows that the thickness tends to increase as the concentration of the critical oil increases; this may be because the essential oil is not fully compatible with the gelatin matrix, increasing the film thickness. In addition, Taqi et al. (2014) 41 mention that essential oils increase the solids present in the solution (partly due to the presence of fatty acids). Therefore, when pouring the same volume of filmogenic solution on the plates, the solids coming from the essential oil will increase the thickness of the polymeric matrix once the drying process has been completed, especially when using the function drying method 38–41 .

Figure 1. Effect of essential oil concentration on the thickness of edible coatings. Different letters indicate a significant difference between the thickness of the treatments (P ≤ 0,05), according to Tukey's HSD test. EO represents the essential oil at various concentrations.
Moisture content
Moisture is a crucial factor to consider when considering edible coatings. High moisture levels can affect the resistance of such coatings, increasing the water content of the food. Ultimately, this can reduce the shelf life of the coated products as it can promote the growth of harmful microbes 42 .
The developed coatings present a moisture range from 16,97 % to 22,72 %, depending on the essential oil content. The moisture content of the control coating was 19.63%, which is lower than the values reported by Caetano et al. (2017) 40 (28,1%) and Medina et al. (2015) 31 (37,9 %). The reduction in the moisture value compared to the previously mentioned studies could be because gelatin interacts with the polymeric chains of starch, reducing the free hydroxyl groups and thus preventing the polymeric matrix from interacting with water molecules.
Unlike the other analyzed parameters, figure 2 shows that moisture does not follow a linear trend. The analysis of variance shows that the control cover does not present significant differences (p<0,05) with the treatment of 1% EO and 5% EO. This implies that the humidity reaches its lowest value at 2,5% of essential oil and its highest value at a concentration of 10% EO.

Figure 2. Effect of essential oil concentration on the moisture content of edible coatings. Different letters indicate a significant difference between the thickness of the treatments (P ≤ 0,05), according to Tukey's HSD test. EO represents the essential oil at various concentrations.
Several investigations suggest that the incorporation of substances such as extracts 18,44 , essences 44, and essential oils 38,42 of plants gradually reduce the percentage of moisture of biodegradable coatings since these substances increase the hydrophobic portion of the matrix, thus reducing the affinity of the polymeric chains for water molecules. It was observed that the humidity of the elaborated coatings decreases with the incorporation of lavender essential oil. However, this tendency is only observed up to the concentration of 2,5% EO. Contrary to what was expected, the concentrations of 5% EO and 10% EO increased humidity relative to the control. This behavior can be evidenced in the study of Desire et al. (2017) 42 in which the addition of 10% essential oil significantly (p <0.01) increases the moisture, causing it to change from 16,57% to 24,34%. Similarly, Bharti et al. (2021) 46 research shows that a high concentration of essential oil (3%) can increase its moisture. The wetting behavior may be because high concentrations of critical oil cause the film to increase in thickness, making it more susceptible to water molecules being trapped within the polymer matrix 46 .
Solubility
The solubility of edible coatings is an important parameter to consider since, for their application in the packaging industry, they must present low solubility and high water resistance 48 . It was observed that coatings without EO present high solubility (62,482%), concerning other research 19,52 . This effect could be due to the incorporation of gelatin in the formulation since this is hydrophilic, as are the different components of the filmogenic solution 49, 21 . However, upon incorporation of EO, a decrease in solubility is denoted as its concentration increases; that is how the solubility is reduced from 62,482% (control coating) to 26,871% at the 10% concentration of essential oil. This trend is also observed in other studies, thus suggesting that incorporating an element of hydrophobic nature into the formulation reduces the formation of molecular interactions between the polymer matrix and water 45,51 . In addition, the oil's non-polar components interact favorably with gelatin's hydrophobic domain, leading to an increase in the hydrophobicity of the resulting film 10.

Figure 3. Effect of the concentration of the essential on the percentage solubility of edible coatings. Different letters indicate a significant difference between the thickness of the treatments (P ≤ 0,05), according to Tukey's HSD test. EO represents the essential oil at various concentrations.
Swelling Capacity
The swelling capacity allows determining the water resistance of the coating, which is essential for its application in the packaging of foods with high water content 50 . The swelling capacity values should remain low, indicating that the coating will not affect its appearance and protective function 42 . The starch-based coatings developed Šuput et al., (2016) 38 had a value of 231%, which is lower than the 381,04% value presented by the control coatings developed in this research. However, Esteghlal et al., (2016) 22 developed gelatin-based coatings with a comparable swelling capacity of 314,16%.
On the other hand, in Figure 4, it can be observed that the swelling capacity tends to decrease with an increase in the concentration of essential oil. These results are consistent with the findings of Šuput et al., (2016) 38, who reported that the swelling capacity decreased from 231% (control) to 118% when a 2% concentration of oregano essential oil was added. According to a study by Davoodi et al., (2017 ) 51, the adding of thymol to gelatin-based coatings reduces the swelling capacity by 42% (from 390% to 348%) when 8% thymol is used. As explained by Di Pierro et al., (2006) 52 the degree of swelling in a polymer matrix is heavily influenced by the amount and type of intermolecular interaction occurring between the polymer chains.
This result was expected considering the decrease in solubility with increasing concentration of the essential oil mentioned above. Furthermore, according to Davoodi et al. (2017) 51, the addition of hydrophilic compounds is expected to increase the swelling capacity, and the addition of hydrophobic compounds such as essential oils generates that the swelling capacity of biodegradable films decreases.

Figure 4. Effect of the essential oil concentration on the swelling capacity of edible coatings. Different letters indicate a significant difference between the thickness of the treatments (P ≤ 0,05), according to Tukey's HSD test. EO represents the essential oil at various concentrations.
Water Vapor Transmission Rate (WVTR) and Water Vapor Permeability (WVP)
Water vapor permeability (WVP) reflects the ability of coatings to control water vapor transmission between food and the environment. Generally, starch-based coatings present high WVP values due to their hydrophilic nature and high porosity 54.
The water vapor transmission rate (WVTR) recorded in the elaborated control group coatings was 15,313 g/m2h. This value is higher than the 8,58 g/m2h observed in the study conducted by Šuput et al., (2016) 38 where starch-based films were used. The increase in WVTR may be attributed to the presence of gelatin in the formulation, which enhances the hydrophilicity of the coatings.
As mentioned, incorporating essential oil in the films reduces the water vapor transmission rate (WVTR). This is because the oils reduce the interactions of the hydrophilic portions of the matrix, which results in fewer water molecules being captured by the films 17 . Additionally, as the thickness of a material increases, the WVTR decreases due to an increase in the material's resistance to water vapor flow. The results of this research are consistent with those presented by Bharti et al., (2021) 46 and Šuput et al., (2016) 38 where an increase in essential oil content reduced the WVTR. Nevertheless, the decrease was significant concerning the control due to the incorporation of 3% essential oil.
The research conducted by Yanwong & Threepopnatkul, (2015) 54, found that the incorporation of 10% peppermint oil in biodegradable covers reduced the water vapor transmission rate (WVTR) from 2,71 g/m2.h to 2,49 g/m2.h. This indicates that the addition of high amounts of essential oil can decrease the WVTR, but not abruptly. Table 2 presents the values of water vapor permeability (WVP) of the biodegradable covers, where the coating with 10% EO resulted in the lowest WVP value of 14,641 x 10-6 g.mm.h-1.Pa-1.m-2.

Table 2. Water Vapor Transmission (WVTR) and Water Vapor Permeability (WVP) of edible coatings based on cassava starch and gelatin incorporated with lavender essential oil. Different letters in the same column indicate a significant difference (P ≤ 0.05)
Antimicrobial activity
Antimicrobial activity is a highly relevant parameter for food preservation during the transport chain. Essential oils, by nature, have antimicrobial activity against various pathogenic strains 37 . Lavender oil primarily comprises terpenic alcohols, with linalool and linalyl acetate being the most prominent 55 . In this particular research, the lavender oil used had linalool (31,77%), linalyl acetate (28,60%), camphor (8,30%), and 1,8 cineole (6,57%). However, these terpenic compounds are lower in concentration than Kwiatkowski et al. (2020) 56 research, which reported values of 34,1% for linalool and 33,3% for linalyl acetate.
Table 3 displays the inhibition halos of the coatings developed against Escherichia coli (ATCC 11229) and Staphylococcus aureus (ATCC 13150) strains. The control and the 1% EO formulation exhibit no antimicrobial activity, as several studies suggest that essential oils do not have antimicrobial properties at low concentrations 24.
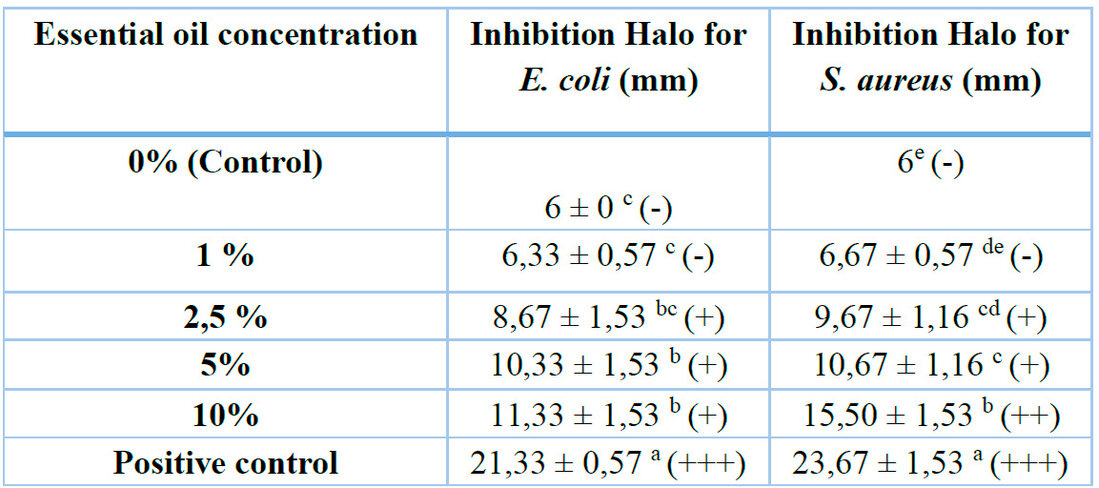
Table 3. Inhibition halos are exhibited by edible coatings enriched with Lavender essential oil. Additionally, different investigations with different essential oils are represented. Other letters in the same column indicate a significant difference in the inhibition halos exhibited by the treatments (p ≤ 0,05). Symbols represent not sensitive (-)for diameters less than 8 mm; sensitive (+)for diameters 9-14 mm; very sensitive (++)for diameters 15-19 mm; extremely sensitive (+++)for diameters more significant than 20 mm. EO corresponds to essential oil. Positive control corresponds to Gentamicin 30 µg/disc.
At concentrations higher than 2.5%, essential oil (EO) can effectively inhibit microbial growth. The concentration of the oil directly influences the degree of inhibition. In the publication of Jamróz et al., (2018) 10, it was observed that at a concentration of 6% lavender essential oil, halos of 15.1 mm and 24.5 mm were obtained for E. coli and S. aureus, respectively. The investigation showed that the highest inhibition value was observed at a 10% concentration of EO, with values of 11.33 mm for E. coli and 15.50 mm for S. aureus. These values are comparatively lower than those reported in the study by Jamróz et al. (2018) 10. This may be due to variations in the origin of the essential oil, its composition, and purity.
In Figure 5, a comparison is shown between the inhibitory effect of the films on E. coli and S. aureus.

Figure 5. Effect of Lavender essential oil concentration on the inhibition halos presented by edible coatings. Different letters indicate a significant difference between the thickness of the treatments (P ≤ 0.05), according to Tukey's HSD test. EO represents essential oil.
It is observed that S. aureus strain is more sensitive to formulations with higher concentrations of lavender oil. This trend has been observed in several investigations where the inhibition halos of E. coli are lower than those of S. aureus 23,24,46,57,58 . The ability of lipoteichoic acids in the cell membrane of gram-positive bacteria (S. aureus) to penetrate hydrophobic components of essential oils might explain this. In contrast, the double membrane of gram-negative bacteria (E. coli) restricts the diffusion rate of hydrophobic compounds through the lipopolysaccharide layer. 10.
Furthermore, several studies have demonstrated that S. aureus is more sensitive than E. coli to different plant extracts, including Zingiber zerumbet, Eucalyptus globulus, and Andrographis paniculata, which have been shown to reduce the biofilm formation and enhance antibiotic sensitivity 60,61 effectively.
Although promising results with potential industrial applications were obtained, it is essential to acknowledge the study's limitations, including the need for more comprehensive microbiological analyses, particularly concerning storage conditions and potential interactions with packaged food. Therefore, this study lays a solid foundation for future research to address these aspects and further refine the parameters evaluated.
CONCLUSIONS
The results suggest that edible coatings are a promising and viable alternative to conventional wrappings, primarily because they are mainly composed of compounds of natural origin, such as polysaccharides and proteins. In addition, bioactive ingredients with antioxidant and antimicrobial properties can be added. In particular, the developed coatings demonstrated an ability to inhibit the growth of common pathogenic bacterial species, suggesting that they can be an effective alternative to protect foods from bacterial contamination and prolong their shelf life.
Thus, this research seeks to establish the basis for developing applied research in food protection. Although it is not a pioneer in this field, its objective is to offer a broad panorama that allows taking advantage of renewable primary sources and active ingredients, such as essential oils or medicinal plant extracts.
Author Contributions: GZ, CS: review of the state of the art, discussion, and composition; IT: design, methodology proposal, comparative results, composition, syntax, and writing of the article; GZ, GV: writing and discussion of the physicochemical characterization status; GV: results and discussion around antimicrobial properties; JA: writing and proofreading of the manuscript.
Funding: This research received no external funding.
Acknowledgments: The authors thank the Research Direction of the Escuela Superior Politécnica de Chimborazo for their support through the project "Evaluation of the leaves and fruit anti-inflammatory activity of mortiño (Vaccinium floribundum Kunth) in vitro in porcine aorta primary endothelial cells (Paecs) and in vivo in mice (Mus musculus) balb/c strain."
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Souza CO, Silva LT, Silva JR, López JA, Veiga-Santos P, Druzian JI. Mango and acerola pulps as antioxidant additives in cassava starch bio-based film. J Agric Food Chem. 2011 Mar 23;59(6):2248–54.
2. Flores S, Famá L, Rojas AM, Goyanes S, Gerschenson L. Physical properties of tapioca-starch edible films: Influence of filmmaking and potassium sorbate. Food Research International. 2007 Mar;40(2):257–65.
3. Hu G, Chen J, Gao J. Preparation and characteristics of oxidized potato starch films. Carbohydr Polym. 2009 Mar 17;76(2):291–8.
4. Bonilla J, Talón E, Atarés L, Vargas M, Chiralt A. Effect of the incorporation of antioxidants on physicochemical and antioxidant properties of wheat starch-chitosan films. J Food Eng [Internet]. 2013 Oct [cited 2021 Apr 19];118(3):271–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0260877413001726
5. Chang-Bravo L, López-Córdoba A, Martino M. Biopolymeric matrices made of carrageenan and corn starch for the antioxidant extracts delivery of Cuban red propolis and yerba mate. React Funct Polym [Internet]. 2014 Dec [cited 2021 19 April];85:11–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1381514814002041
6. Piñeros-Hernandez D, Medina-Jaramillo C, López-Córdoba A, Goyanes S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017 Feb 1;63:488–95.
7. Daza LD, Homez-Jara A, Solanilla JF, Váquiro HA. Effects of temperature, starch concentration, and plasticizer concentration on the physical properties of ulluco (Ullucus tuberosus Caldas)-based edible films. Int J Biol Macromol. 2018 Dec 1;120:1834–45.
8. Khodaei D, Oltrogge K, Hamidi-Esfahani Z. Preparation and characterization of blended edible films manufactured using gelatin, tragacanth gum and, Persian gum. LWT. 2020 1 January;117.
9. Rojas-Graü MA, Avena-Bustillos RJ, Friedman M, Henika PR, Martín-Belloso O, Mchugh TH. Mechanical, barrier, and antimicrobial properties of apple puree edible films containing plant essential oils. J Agric Food Chem. 2006;54(24):9262–7.
10. Jamróz E, Juszczak L, Kucharek M. Investigation of the physical properties, antioxidant and antimicrobial activity of ternary potato starch-furcellaran-gelatin films incorporated with lavender essential oil. Int J Biol Macromol. 2018 Jul 15;114:1094–101.
11. Paparella A, Maggio F. Detection and Control of Foodborne Pathogens. Foods 2023, Vol 12, Page 3521 [Internet]. 2023 22 September [cited 2024 14 March];12(19):3521. Available from: https://www.mdpi.com/2304-8158/12/19/3521/htm
12. Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils – A review. Food and Chemical Toxicology. 2008 Feb 1;46(2):446–75.
13. Manion CR, Widder RM. Essentials of essential oils. American Journal of Health-System Pharmacy [Internet]. 2017 May 1 [cited 2024 Mar 14];74(9):e153–62. Available from: https://dx.doi.org/10.2146/ajhp151043
14. Gismondi A, Di Marco G, Redi EL, Ferrucci L, Cantonetti M, Canini A. The antimicrobial activity of Lavandula angustifolia Mill. essential oil against Staphylococcus species in a hospital environment. J Herb Med. 2021 Apr 1;26:100426.
15. Moussi Imane M, Houda F, Said Amal AH, Kaotar N, Mohammed T, Imane R, et al. Phytochemical Composition and Antibacterial Activity of Moroccan Lavandula angustifolia Mill. Journal of Essential Oil Bearing Plants [Internet]. 2017 Jul 4 [cited 2024 Mar 14];20(4):1074–82. Available from: https://www.tandfonline.com/doi/abs/10.1080/0972060X.2017.1363000
16. ISO 6647-1:2020. ISO 6647-1:2020(en), Rice — Determination of amylose content FORMATO DIDE-PRY-002-2017 15 — Part 1: Spectrophotometric method with a defatting procedure by methanol and with calibration solutions of potato amylose and waxy rice amylopectin. 2020.
17. Medina C, Goyanes S, Bernal C. Biofilms based on cassava starch containing extract of yerba mate as antioxidant and plasticizer. 2015;(April 2018).
18. Medina C, Gutiérrez TJ, Goyanes S, Bernal C, Famá L. Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydr Polym. 2016;151:150–9.
19. Ashwar BA, Shah A, Gani A, Shah U, Gani A, Wani IA, et al. Rice starch active packaging films loaded with antioxidants-development and characterization. Starch/Staerke. 2015;67(3–4):294–302.
20. Thakur R, Saberi B, Pristijono P, Golding J, Stathopoulos C, Scarlett C, et al. Characterization of rice starch-ι-carrageenan biodegradable edible film. Effect of stearic acid on the film properties. Int J Biol Macromol. 2016;93:952–60.
21. Tongdeesoontorn W, Mauer LJ, Wongruong S. Mechanical and Physical Properties of Cassava Starch-Gelatin Composite Films. International Journal of Polymeric Materials and Polymeric Biomaterials. 2012;(September 2013):37–41.
22. Esteghlal S, Niakosari M, Hosseini SMH, Mesbahi GR, Yousefi GH. Gelatin-hydroxypropyl methylcellulose water-in-water emulsions as a new bio-based packaging material. Vol. 86, International Journal of Biological Macromolecules. Elsevier BV; 2016. 242–249 p.
23. Pelissari F, Grossmann M, Yamashita F, Pineda E. Antimicrobial , Mechanical , and Barrier Properties of Cassava Starch - Chitosan Films Incorporated with Oregano Essential Oil. 2009;7499–504.
24. Debiagi F, Kobayashi RKT, Nakazato G, Panagio LA. Biodegradable active packaging based on cassava bagasse , polyvinyl alcohol and essential oils. Ind Crops Prod. 2014;52:664–70.
25. Weintein M, Lewis J, Bobenchik A, Campeau S, Cullen S, Galas M, et al. Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. Clinical and Laboratory Standards Institute (CLSI); 2020.
26. Aristizábal J, Sánchez T. Guía técnica para producción y análisis de almidón de yuca. Boletín de Servicios Agrícolas Fao. 2007;163:134.
27. Navia Porras DP, Gordillo Suárez M, Hernández Umaña J, Poveda Perdomo LG. Optimization of Physical, Optical and Barrier Properties of Films Made from Cassava Starch and Rosemary Oil. J Polym Environ. 2019;27(1):127–40.
28. Souza AC, Goto GEO, Mainardi JA, Coelho ACV, Tadini CC. Cassava starch composite films incorporated with cinnamon essential oil: Antimicrobial activity, microstructure, mechanical and barrier properties. LWT - Food Science and Technology. 2013;54(2):346–52.
29. Da Silva JBA, Pereira F V., Druzian JI. Cassava Starch-Based Films Plasticized with Sucrose and Inverted Sugar and Reinforced with Cellulose Nanocrystals. J Food Sci. 2012 Jun;77(6).
30. Piñeros-hernandez D, Medina-jaramillo C, López-córdoba A, Goyanes S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. 2016;
31. Medina Jaramillo C, Gutiérrez TJ, Goyanes S, Bernal C, Famá L. Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydr Polym. 2016;151:150–9.
32. Charro M. Obtención de Plásticos Biodegradables a partir de Patata. [Quito]: Universidad Central Del Ecuador; 2015.
33. He R, Fu NF, Chen HM, Ye JQ, Chen LZ, Pu YF, et al. Comparison of the structural characterizatics and physicochemical properties of starches from sixteen cassava germplasms cultivated in China. Int J Food Prop. 2020 Jan 1;23(1):693–707.
34. Mohamed SAA, El-sakhawy M, El-sakhawy MA monem. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Elsevier. 2020;116178.
35. Al-Hassan AA, Norziah MH. Starch-gelatin edible films: Water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocoll. 2012;26(1):108–17.
36. Santana O, Cabrera R, Herraiz D. Perfil químico y biológico de aceites esenciales de plantas aromáticas de interés agro-industrial en Castilla-La Mancha ( España ). 2012;63(2):214–22.
37. Romeo F V., De Luca S, Piscopo A, Poiana M. Antimicrobial effect of some essential oils. Journal of Essential Oil Research. 2008;20(4):373–9.
38. Šuput D, Lazić V, Pezo L, Markov S, Vaštag Ž, Popović L, et al. Characterization of starch edible films with different essential oils addition. Pol J Food Nutr Sci. 2016;66(4):277–85.
39. Supardan MD, Annisa Y, Arpi N, Aida W, Mustapha W. Cassava Starch Edible Film Incorporated with Lemongrass Oil : Characteristics and Application. 2016;(April).
40. Caetano K dos S, Hessel CT, Tondo EC, Flôres SH, Cladera-Olivera F. Application of active cassava starch films incorporated with oregano essential oil and pumpkin residue extract on ground beef. J Food Saf. 2017;37(4):1–9.
41. Taqi A, Mutihac L, Stamatin I. Physical and barrier properties of apple pectin/cassava starch composite films incorporating laurus nobilisl. oil and oleic acid. J Food Process Preserv. 2014;38(4):1982–93.
42. Desire A, Charlemagne N, Achille T, Catherine D, Georges A, Marianne S. Water Vapor Permeability of Edible Films Based on Improved Cassava (Manihot esculenta Crantz) Native Starches. J Food Process Technol. 2017;08(03).
43. Apriliyani M, Purwadi P, Manab A, Ikhwan A. Characteristics of Moisture Content, Swelling, Opacity and Transparency with Addition Chitosan as Edible Films/Coating Base on Casein. Advance Journal of Food Science and Technology. 2020 Apr 25;18(1):9–14.
44. Riaz A, Lagnika C, Luo H, Dai Z, Nie M, Hashim MM, et al. Chitosan-based biodegradable active food packaging film containing Chinese chive (Allium tuberosum) root extract for food application. Int J Biol Macromol. 2020;150:595–604.
45. Moosavian V, Mehdi Marvizadeh M, Nafchi AM. Biodegradable Films Based on Cassava Starch/Mentha piperita Essence: Fabrication, Characterization and Properties. Journal of Chemical Health Risks. 2017;7(3):239–45.
46. Bharti SK, Pathak V, Alam T, Arya A, Singh VK, Verma AK, et al. Starch bio-based composite active edible film functionalized with Carum carvi L. essential oil: antimicrobial, rheological, physic-mechanical and optical attributes. J Food Sci Technol. 2021;
47. Rojhan M, Nouri L. Antimicrobial , Physicochemical , Mechanical , and Barrier Properties of Tapioca Starch Films Incorporated with Eucalyptus Extract. Journal of Chemical Health Risks. 2013;3(3):43–52.
48. Guerrero-beltrán MCV briones JA. Recubrimientos de frutas con biopelículas. 2013;5–14.
49. Moosavian V, Mehdi Marvizadeh M, Nafchi AM. Biodegradable Films Based on Cassava Starch/Mentha piperita Essence: Fabrication, Characterization and Properties. Journal of Chemical Health Risks. 2017;7(3):239–45.
50. Galus S, Kadzińska J. Moisture Sensitivity, Optical, Mechanical and Structural Properties of Whey Protein-Based Edible Films Incorporated with Rapeseed Oil. Food Technol Biotechnol. 2016 Jan 1;54(1):78.
51. Davoodi M, Kavoosi G, Shakeri R. Preparation and characterization of potato starch-thymol dispersion and film as potential antioxidant and antibacterial materials. Int J Biol Macromol. 2017 Nov 1;104:173–9.
52. Di Pierro P, Chico B, Villalonga R, Mariniello L, Damiao AE, Masi P, et al. Chitosan-whey protein edible films produced in the absence or presence of transglutaminase: Analysis of their mechanical and barrier properties. Biomacromolecules. 2006;7(3):744–9.
53. Valencia-Sullca C, Vargas M, Atarés L, Chiralt A. Thermoplastic cassava starch-chitosan bilayer films containing essential oils. Food Hydrocoll. 2018;75:107–15.
54. Yanwong S, Threepopnatkul P. Effect of peppermint and citronella essential oils on properties of fish skin gelatin edible films. IOP Conf Ser Mater Sci Eng. 2015;87(1).
55. Cavanagh HMA, Wilkinson JM. Lavender essential oil: a review. Australian Infection Control. 2005;10(1):35–7.
56. Kwiatkowski P, Łopusiewicz Ł, Kostek M, Drozłowska E, Pruss A, Wojciuk B, et al. The antibacterial activity of lavender essential oil alone and in combination with octenidine Dihydrochloride against MRSA strains. Molecules. 2020;25(1).
57. Predoi D, Iconaru SL, Buton N, Badea ML, Marutescu L. Antimicrobial activity of new materials based on lavender and basil essential oils and hydroxyapatite. Nanomaterials. 2018;8(5).
58. Cruz-Tirado JP, Barros Ferreira RS, Lizárraga E, Tapia-Blácido DR, Silva NCC, Angelats-Silva L, et al. Bioactive Andean sweet potato starch-based foam incorporated with oregano or thyme essential oil. Food Packag Shelf Life.2020;23(December 2019):100457.
59. Pirzada BKM, Tajammul A, Ahmed Z. The Disinfectant Properties of Suspensions of Herbal Extracts: An Antibacterial Study against Escherichia Coli and Staphylococcus Aureus. Journal of Sustainable Environmental. 2022 Jun 19;1(1):18–23.
60. Elisabeth Zeuko’o Menkem, Rufin Marie Toghueo Kouipou, Cedric Derick Jiatsa Mbouna, Maguerite Simo Kamdem, Patrick Valere Tsouh Fokou, Fekam Boyom Fabrice. Antibacterial Screening of Fifteen Cameroonian Medicinal Plants against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Salmonella typhi. Journal of Pharmacy and Pharmacology. 2016 Oct 28;4(10)
Received: 4 August 2024 / Accepted: 3 September 2024 / Published: 15 September 2024
Citation: Tubon I, Zambrano-Cárdenas GO, Arcos-Logroño JP, Santiana-Espín CG, Vaca-Altamirano GL. Innovative protection: characterization of edible cassava and lavender coatings for sustainable packaging solutions. Bionatura journal. 2024;1(3):20. doi: 10.70099/BJ/2024.01.03.19
Additional information Correspondence should be addressed to itubon@espoch.edu.ec
Peer review information. Bionatura thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
ISSN.3020-7886
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Publisher's Note: Bionatura Journal stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2024 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


