Antagonistic activity of biocontrol agent Trichoderma spp. against Fusarium sp., the causal agent of Ananas comosus fruitlet rot
Lucas Martín Madrassi 1,3*, Adriana Elizabet Alvarenga1,3, María Celina Vedoya 2,
1 Universidad Nacional de Misiones. Facultad de Ciencias Exactas, Químicas y Naturales. Instituto de Biotecnología Misiones "Dra. María Ebe Reca" (INBIOMIS). Laboratorio de Biotecnología Molecular (BIOTECMOL). Ruta Nacional 12 Km 7,5, C.P. 3300, Misiones, Argentina;
2 Universidad Nacional de Misiones. Facultad de Ciencias Exactas, Químicas y Naturales. Laboratorio de Micología “Dra. Martha G. Medvedeff”. Av. Mariano Moreno 1375, C.P. 3300, Misiones, Argentina;
3 Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). Godoy Cruz 2290, C.P. 1650. CABA, Argentina.
* Correspondence: lmmadrassi@hotmail.com
ABSTRACT
Pineapple (Ananas comosus) is a significant crop, with an annual production exceeding 25 million tons. However, fusariosis can severely impact its cultivation, a fungal disease that causes fruitlet rot and results in substantial yield losses. To decrease dependency on chemical control methods, biocontrol agents (BCAs) present a promising alternative. Among these, Trichoderma species are noteworthy due to their diverse antagonistic mechanisms. The efficacy of each mechanism can be assessed through fungal confrontation assays. This study aimed to isolate, identify, and evaluate in-vitro nine Trichoderma spp. strains as potential BCAs against Fusarium sp. associated with pineapple fruitlet rot. The antagonistic fungi were isolated from rhizosphere soils in both open-field and greenhouse pineapple farms in Misiones province, Argentina. Identification of the fungi required both morphologic and genetic data. In the in-vitro assays, the capabilities for direct competition for substratum, production of metabolites, and mycoparasitism were evaluated. The results indicated that isolates T. harzianum TC7, T. harzianum TC9, T. asperellum TU3, and T. asperellum TU4 had statistically superior inhibitory effects against Fusarium sp. These isolates can be potentially used in formulating natural fungicides to reduce pineapple fruitlet rot caused by Fusarium, promoting sustainable production practices.
Keywords: pineapple, confrontation, mycoparasitism, metabolites, ITS region
INTRODUCTION
The excessive or inadequate use of pesticides in tropical and subtropical crops can result in economic, social and environmental harm1. Furthermore, pesticide-mediated control of microorganisms is often inefficient, as these organisms may develop resistance". In tropical and subtropical regions, higher frequencies of pesticide applications are required to manage microorganism-related diseases, such as fungal infections caused by Fusarium2. Fusariosis in both open-field and greenhouse pineapple farms is particularly problematic, as it is associated with fruitlet rot, leading to severe yield losses and significant economic costs 3–5.
Concerns regarding chemical-based control have driven the development of alternative approaches6, such as integrated crop management (ICM) strategies7. A key component of ICM is biocontrol agents (BCAs), also known as biopesticides8. These can be applied on farms as natural antagonists of pests. The use of BCAs is considered environmentally friendly and harmless to human health9.
Most investigations concerning BCAs have focused on Trichoderma species due to their multiple antagonistic mechanisms, which act synergistically to control plant diseases. Trichoderma species exhibit high growth and reproductive rates, survival across various environmental conditions, nutrient consumption and competition efficiency, and ability to act as necrotrophs against other fungi.
In practice, morphological classification must be complemented with molecular data, for which multiple deoxyribonucleic acid (DNA) loci analysis is required. In particular, the Internal Transcribed Spacer region (ITS), within ribosomal DNA serves as a reference locus for identifying Trichoderma species10.
As an initial step in evaluating a Trichoderma isolate as a BCA, qualitative in-vitro assays are necessary. These trials are predictive tools to determine mycelial growth inhibition against specific pathogens. It is possible to separately analyze the antagonistic mechanisms of direct competition for substrate, mycoparasitism, and the production of fungistatic compounds, known as metabolites11,12. As the final steps in this evaluation, it is necessary to investigate the most promising isolates in planta assays under controlled or field conditions and develop mass production techniques for the biopesticide.
This work aimed to isolate, identify, and evaluate in-vitro the potential of native Trichoderma isolates as BCAs against Fusarium sp., associated with pineapple fruitlet rot.
MATERIALS AND METHODS
Isolation of rhizosphere Trichoderma strains
Rhizospheric soil samples were collected from 9 pineapple farms in Argentina, and two farming systems were considered: open-field and greenhouse cultivation (see Table 1). 100g of soil was collected from each farm, mixed, and homogenized manually. Fungal colonies were obtained using serial dilution and cultured on Trichoderma Selective Medium (TSM)13,14 at 28±2ºC for 7 days. Trichoderma colonies were individually subcultured on Potato Dextrose Agar (PDA) at 28±2ºC for 7 days.

Table 1. Information on the Trichoderma isolates, including the type of farming system (open-field or greenhouse cultivation), region, and town of the pineapple farms
Morphological identification of the Trichoderma isolates
Monosporic colonies were obtained for further proposals. These were cultivated in PDA and Malt Extract Agar at 28±2°C, in darkness, for 15 days. Regular observations of macroscopic characteristics, including color, shape, pigment production or liberation, and growth rate, were recorded. Microscopic examination involves the identification of vegetative and reproductive structures according to 15 using an optical microscope (Olympus, CX23).
DNA extraction, amplification, and sequence analysis
Genomic DNA was extracted according to 16. The ITS1-5.8S-ITS2 region was amplified using the polymerase chain reaction (PCR) technique according to 17. Macrogen Inc. (Seoul, Korea) standard sequencing services purified and sequenced PCR products. The quality of DNA sequencing was verified using Chromas Lite v2.1, and sequences were manually trimmed using BioEdit v7.218. Subsequently, the sequences underwent an essential local alignment search tool for nucleotides (BLASTn) analysis against the National Centre for Biotechnology Information (NCBI) database. Multiple sequence alignment was performed using AliView v1.2819 with the ClustalW method.
Phylogenetic analysis of ITS1-5.8S-ITS2 region
Phylogenetic inference was conducted using the Bayesian method. All characters were treated as equally weighted, with gaps considered missing data. The results from the BLASTn submission were downloaded, verified and selected for phylogenetic reconstruction (sequences are listed in Table 2). The outgroup in the inference was Clonostachys spp., with C. rosea CBS 154.27 and C. solani CBS 183.30 (NCBI database Reference Sequence, accession numbers: NR_165993 and NR_163540, respectively).
The find-best-fit-model function was performed in Mega v720 to identify the most informative phylogenetic model. With the resulting model, the Maximum Likelihood method with a bootstrap of 1000 repetitions was applied. The tree was visualized and edited with the same software.

Table 2. The taxonomic identification, isolate name, accession number in the NCBI database, and publication or reference are listed for each sequence used in the phylogenetic reconstruction of the ITS1-5.8S-ITS2 region of the native Trichoderma isolates and sequences from NCBI database
Antagonism potential of rhizosphere Trichoderma, in-vitro
In-vitro assays were made in Petri dishes with PDA at 28±2°C in darkness for 10 days. The pathogenic Fusarium sp. strain used in these assays was obtained from the culture collection of the Laboratory of Mycology "Dra. Martha G. Mevdeveff" from the "Facultad de Ciencias Exactas, Químicas y Naturales, UNaM". This strain was isolated on a diseased pineapple plant from Apostoles City, Misiones province. Negative controls consisted of identical assays but without Trichoderma being inoculated.
Mycoparasitic activity determination
The mycoparasitic activity was confirmed by directly observing cellular interactions between the Trichoderma and Fusarium isolates. The interactions between the colonies were investigated using the microculture method 21 with slight modifications 22, and the colonies were cultivated in 0.1ml of PDA for 5 days. During observation with an optical microscope, the mycelium was stained with lactophenol-cotton-blue to enhance visualization and examined using 40X and 100X magnifications.
Estimation of the growth inhibition of Fusarium sp. by Trichoderma
The antagonistic activity was estimated as %I=(C-T/C)x100, where %I is the percentage of mycelial growth inhibition and T and C are the mean Fusarium colonies radius in the treatments (with Trichoderma) and the controls (without Trichoderma), respectively. Three biological replicates were performed for each treatment.
Direct confrontation (DC)
%I was measured in the DC experiments according to 23. In this case, 5mm discs of Trichoderma and Fusarium were deposited oppositely at 5mm of the edge of a single Petri dish. We also used the antagonism scale (from 1 to 5) proposed by 24.
Indirect confrontation by diffusible (ID) and volatile (IV) metabolites
This study evaluated two types of indirect confrontation assays: diffusible metabolites (ID) and volatile metabolites (IV).
The ID production experiments were carried out as per 11. 5mm discs of Trichoderma were plated on sterile PDA plates, and the medium surface was covered with a layer of cellulose paper. Trichoderma strains were grown for 2 days. Afterward, the Trichoderma colonies were carefully removed, and a 5mm disc of Fusarium was plated on the medium.
The IV production experiments followed the protocol outlined in 12. 5mm discs of Trichoderma and Fusarium were plated on separate PDA dishes. The plates were overlaid, with Trichoderma at the bottom and Fusarium at the top. The junction was sealed with parafilm. The technique was modified to visualize Fusarium sp. colonies exposed to Trichoderma volatiles as per 25.
Statistical analysis of the growth inhibition
The antagonistic activities were compared with one-way ANOVA to find the statistically significant inhibitions. Tukey's test was implemented for the pairwise comparisons. Data analysis and visualization were performed using GraphPad Prism v826.
RESULTS
Isolation of rhizosphere Trichoderma strains
Nine fungal isolates with the classic Trichoderma characters were obtained from the rhizosphere soils of pineapple plants from farms located in Misiones province. Six isolates were obtained from open-field farms and named TU1, TU2, TU3, TU4, TU5, and TU6. The remaining three isolates were obtained from greenhouse farms and named TC7, TC8, and TC9. All fungal isolates were maintained and stored in PDA at 4ºC.
Morphological identification of the Trichoderma isolates
Colonies of Trichoderma spp. were initially white for the first 48-72 hours (early mycelium), later turning dark or light green (late mycelium), with scarce aerial mycelia. No diffusible pigment was observed when Trichoderma isolates were cultured alone. Conidial production was observed after 4 days of incubation, with denser conidia in the center of the colonies. Older colonies formed 1-3 concentric rings. Conidiophores were symmetrical, with a uniform central axis from which paired secondary axes emerged. Phialides were slender, hooked, and flask-shaped. Conidia were dark green and globose, appearing after 48-72 hours. Chlamydospores were green and globose, appearing after 5-7 days. We identified these isolates as members of the genus Trichoderma based on morphological characteristics.
During the late mycelium state, the growth rate and sporulation pattern were slightly different between Trichoderma isolates depending on the type of farming system (open-field or greenhouse cultivation) from which they were collected.
Phylogenetic study of the ITS1-5.8S-ITS2 region
The two main groups formed consisted of sequences from the T. harzianum and T. asperellum species complexes (see Fig. 1). These groups had significant statistical support, with bootstrap values higher than 70. The sequences of isolates TU1, TU2, TU3, TU4, TU5, and TU6 grouped with T. asperellum species-complex sequences from NCBI. The sequences of the isolates TC7, TC8, and TC9 grouped with T. harzianum species-complex.
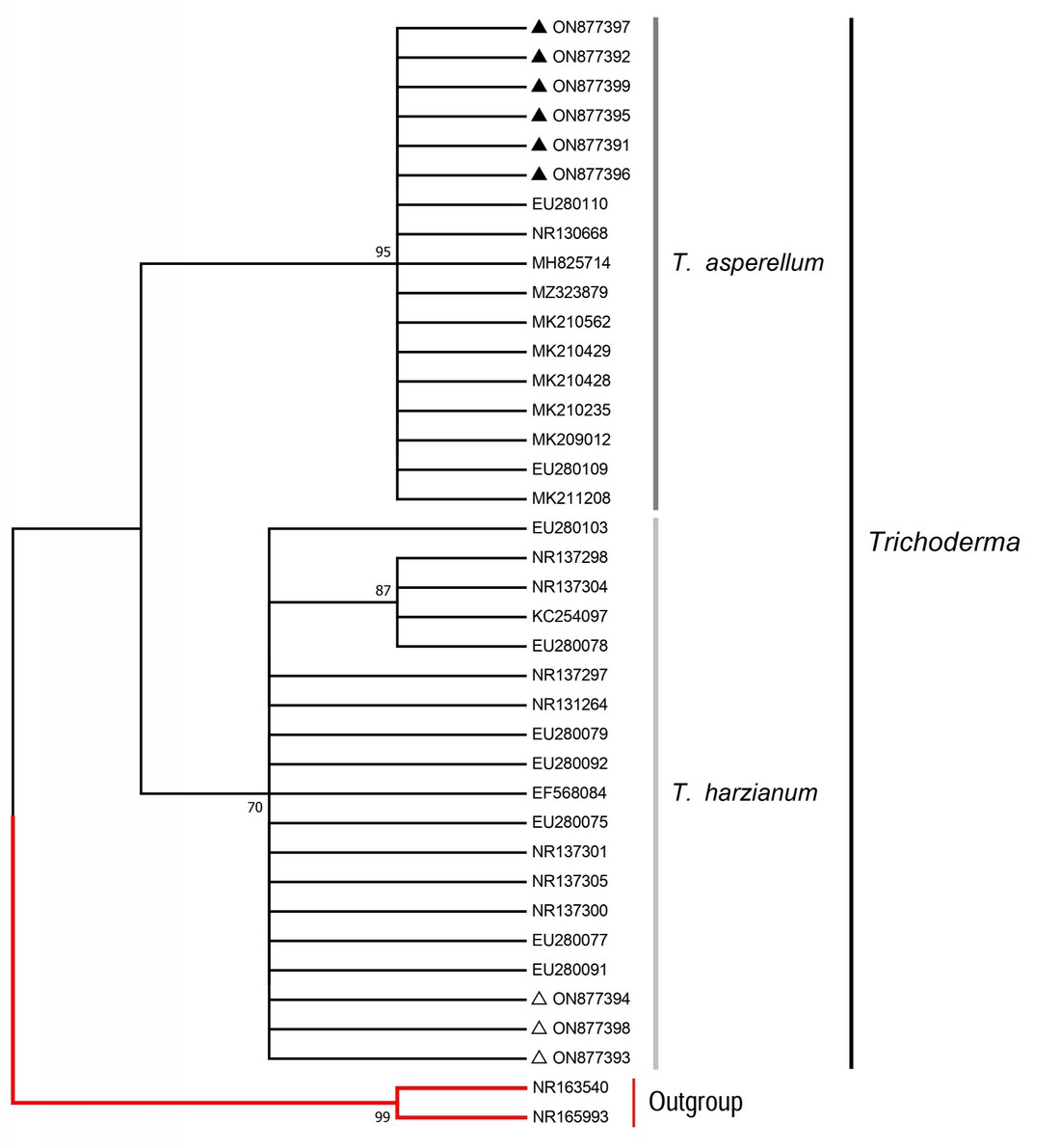
Figure 1. Phylogenetic reconstruction of the ITS1-5.8S-ITS2 region. The tree was constructed using the Maximum Likelihood method with the Jukes-Cantor model, and bootstrap support values were generated from 1000 replicates. Only bootstrap supports equal to or higher than 70 are shown. The isolates from this work are indicated by black triangles (▲) for open-field cultivation and white triangles (△) for greenhouse cultivation. The outgroup (Clonostachys spp.) is colored in red. The vertical bars indicate sequences from the same Trichoderma species complex. These groups are supported by bootstrap values of 70 for the T. harzianum species-complex isolates and 95 for the T. asperellum species-complex isolates.
Mycoparasitic activity determination
The mycoparasitic capability of all the Trichoderma isolates was confirmed by observing the microscopic interaction of the hyphae. These interactions included cellular adhesion, coiling, and penetration toward Fusarium sp. (Fig. 2).

Figure 2. Microphotography of a microculture assay between the isolate T. asperellum TU2 (T) and Fusarium sp. (F), associated with pineapple fruitlet rot. The image shows multiple coiling of Trichoderma towards the Fusarium hyphae. Scale: 20um.
Estimation of the growth inhibition of Fusarium sp. by Trichoderma
The antagonistic strains exhibited a remarkable inhibitory effect against the Fusarium isolate after 10 days of cultivation. The percentage of mycelial growth inhibition (%I) varied among the antagonistic treatments tested. In other words, the inhibition effect against Fusarium sp. varied among the different assays involving Trichoderma isolates (see Table 3). The average colony radius for the Fusarium sp. isolates, without Trichoderma, was 39±2,4mm across three biological replicates after 10 days of cultivation.

Table 3. PICP values for nine Trichoderma isolates against Fusarium sp. in dual culture (DC) and diffusible (IV) and volatile (IV) metabolites assays. Values are shown as mean ± standard deviation. Absolute values for the Fusarium colonies are detailed in Table S1.
Direct confrontation (DC)
The T. harzianum isolates showed the highest antagonistic activity in the DC cultures. In this case, the inhibition ranged from 63 to 69.3%. The inhibition obtained by the T asperellum isolates ranged from 51.7 to 66.3%.
The Trichoderma isolates showed high scores on Bell's scale, with T. asperellum and T. harzianum isolates scoring 1 and 2, respectively. At the end of the 10th day, they exhibited moderate to high overgrowth on the Fusarium sp. colonies.
In all the DC plates tested, the contact zone between the fungi exhibited a curved shape, with the concavity orientated towards the Fusarium isolate. The T. asperellum isolates produced inhibition halos (3-5mm wide) between both colonies, indicating the presence of diffusible metabolite production. The T. harzianum isolates emanated a profuse coconut-like smell, a classical indicator of volatile metabolite production in Trichoderma species27–29.
Indirect confrontation by diffusible (ID) and volatile (IV) metabolites
In ID production assays, T. asperellum isolates showed the highest antagonistic activity. In this case, inhibition ranged from 43.3 to 57.6%. In contrast, inhibition for T. harzianum isolates was weaker for this assay and ranged from 43.7 to 48.7%.
The T. harzianum isolates obtained the strongest inhibitions against Fusarium sp in IV cultures. The %I values ranged from 51.3 to 60.3%. The inhibition for T. asperellum isolates was lower and ranged from 39 to 49.7%.
Remarkably, in the IV assays, the Trichoderma colonies had a yellow coloration after 10 days of cultivation, and the Fusarium isolate did not produce any pigments in the presence of the volatile metabolites produced by Trichoderma spp., so the late mycelia of the colonies remained white (Fig. 3).

Figure 3. Illustrative picture of macro-morphological changes of the Fusarium colonies in controls and treatments within the IV assays. In (a), the Fusarium colony exhibits the typical red pigment produced during cultivation on potato dextrose agar. In (b), the Fusarium colony is exposed to the Trichoderma volatile compounds and does not produce any observable pigments, resulting in a white appearance after 10 days of cultivation. Scale: (a, b): 5mm
Statistical analysis of the growth inhibition
The one-way ANOVA test revealed statistically significant differences in the inhibition values among some of the Trichoderma isolates for each type of the antagonism mechanisms tested (see Fig. 4). Particularly, in the DC experiments (F= 133.3, df= 26, P <0.0001), T. harzianum TC7 was statistically superior to other isolates (Tukey, P<0.05). In the ID assays (F= 59.2, df= 26, P <0.0001), T. asperellum TU3 and T. asperellum TU4 had a statistically different inhibitory effect on Fusarium sp. (Tukey, P<0.05) but not between each other (Tukey, P≥0.5). In the IV assays (F= 147.3, df= 26, P <0.0001), T. harzianum TC9 produced a statistically higher inhibition than any other isolate (Tukey, P<0.05).

Figure 4. The values of %I (vertical axis) of the nine Trichoderma isolates (horizontal axis) against Fusarium sp., associated with pineapple fruitlet rot, in DC (dark bars), ID (grey bars), and IV (white bars) assays. For each %I value, the 95% confidence intervals are shown. The asterisks (*) indicate statistically higher inhibitions (Tukey, P<0,05) for the dual culture (*DC), diffusible metabolites (*ID), and volatile metabolites (*IV) assays.
DISCUSSION
Isolation of rhizosphere Trichoderma strains
The reports suggest that Trichoderma30 and Fusarium5 are prevalent fungal genera in pineapple farms. Specifically, T. harzianum and T. asperellum are frequently isolated in tropical and subtropical environments31, which comprise the primary geographic zones for pineapple cultivation.
Morphological and molecular identification of the Trichoderma isolates
In the present assay, two groups were delineated based on Trichoderma morphological characters. Each group presented the typical morphotypes reported for the T. asperellum species-complex32 and the T. harzianum species-complex isolates33. However, it should be noted that these morphotypes serve as broad descriptors.
On average, the analysis of the ITS1-5.8S-ITS2 region leads to an accurate taxonomic fungal species identification, particularly within the ITS1 and the ITS2 introns34. The formation of phylogenetically significant subgroups within specific species is standard, as observed in 35 and 17, where the T. harzianum isolates were grouped in two or more "clades." Similarly, in the experiments by 32, the T. asperellum isolates formed various subgroups. The two Trichoderma morphotypes in this assay corresponded to distinct ITS1-5.8S-ITS2 phylogenetic groups. The sequences of the T. harzianum species-complex isolates formed subgroups with statistically significant bootstrap values.
However, taxonomic identification at the species level in Trichoderma necessitates the utilization of additional DNA markers36. For example, the Translational Elongation Factor 1α (TEF1α)37 and the RNA Polymerase II Second Largest Subunit (RPB2)38 have been used to distinguish species within the T. asperellum39 and T. harzianum complexes40.
Mycoparasitic activity determination
The ability to parasitize other fungi is widespread among species of the genus Trichoderma. T. asperellum and T. harzianum are no exceptions, as their mycoparasitic capacity has been validated against numerous phytopathogens35.
In this research, the ability of native T. asperellum and T. harzianum isolates to mycoparasitize Fusarium sp. associated with pineapple fruitlet rot was demonstrated. These results are similar to those obtained by 41, where most Trichoderma isolates exhibited the capacity to mycoparasitic Fusarium spp., showing a range of antagonist-pathogen cellular interactions, including adhesion, coiling, and penetration of host hyphae.
Direct confrontation (DC)
The autochthonous T. asperellum and T. harzianum isolates inhibited the mycelial growth of Fusarium sp. by at least 50 and 60%, with maximum %I values of 66.3% (TU2) and 69.3% (TC7), respectively. These %I values are similar to those previously reported by 7 for T. asperellum 33,42 for T. harzianum, with most isolates exhibiting medium to high antagonistic activity in dual cultures. Nevertheless, %I values documented in the literature range from 20% to 70% for T. asperellum and 30% to 90% for T. harzianum. Consistent with the findings in this study, the highest dual culture %I values for T. harzianum isolates were also reported by 42.
The highest %I values were primarily obtained in the DC tests, compared to other assays. This is logical, considering that under these conditions, multiple antagonistic mechanisms could be present simultaneously, such as producing diffusible and volatile metabolites, competition for the substrate, and mycoparasitism, etc43.
Indirect confrontation by diffusible (ID) and volatile metabolites (IV)
The production of diffusible metabolites has been demonstrated in several Trichoderma species, including T. asperellum44 and T. harzianum45. In the present work, the native T. asperellum and T. harzianum isolates produced diffusible substances with fungistatic action against Fusarium sp. in-vitro. This was especially remarkable for the T. asperellum isolates, which exhibited %I values greater than 50%. Moreover, the presence of inhibition halos during the dual cultures may be attributed to these isolates' high production of diffusible compounds.
Similar to the previous case, the ability to produce volatile metabolites has been demonstrated for T. asperellum46 and T. harzianum47. The %I values obtained by the Trichoderma isolates in this assay suggest the production of volatile compounds capable of inhibiting Fusarium sp. mycelial growth. This was particularly notable for T. harzianum isolates, which achieved %I values greater than 50% in this assay. Remarkably, these fungi emitted an intense coconut-like smell during cultivation with Fusarium spp., a scent previously associated with a Trichoderma volatile antibiotic12,27–29.
Utilization of Trichoderma spp. as BCAs in pineapple farms
Our study provides preliminary insights into the presence of T. harzianum and T. asperellum species complexes and their mechanism for controlling Fusarium sp., the causal agent of pineapple fruitlet rot. In the present work, the antagonistic effects of the Trichoderma isolates varied within isolates from the same species complex. The interactions of fungal isolates are considered strain-specific 48,49. In this context, the interactions of novel Trichoderma isolates against multiple pathogenic Fusarium strains associated with pineapple fruitlet rot could improve our understanding of the behavior of BCAs under different conditions.
While promising Trichoderma isolates were identified and evaluated in this work, further research on the formulations and applications of these BCAs for field treatments is necessary50. The effectiveness of Trichoderma isolates in protecting pineapple plants from Fusarium infection should be evaluated in planta to represent a more realistic scenario51. Future research in these areas will enhance the development of robust biocontrol strategies using Trichoderma species against Fusarium-induced fruitlet rot in pineapples under both open-field and greenhouse cultivation in Misiones farms using ICM strategies.
CONCLUSIONS
This work has studied the biocontrol mechanisms of Trichoderma strains belonging to different species groups. Furthermore, the Trichoderma isolates from healthy pineapple rhizosphere soil displayed robust antagonistic activity against Fusarium sp. These results showed that native Trichoderma spp. could reduce the mycelial growth of Fusarium sp., associated with pineapple fruitlet rot.
This is the first report on the isolation, identification, and characterization of antagonistic Trichoderma species from rhizosphere soil in open-field and greenhouse-cultivated pineapple farms in Misiones, Argentina. The antagonistic activity of T. asperellum and T. harzianum species complexes highlights the potential of using these BCAs to formulate natural and highly effective fungicides. Different formulation and application methods must be studied to gain further insight into the utility of Trichoderma as BCA. These isolates might be integrated with other management strategies to reduce pineapple fruitlet rot caused by Fusarium under a sustainable production practice.
Supplementary Materials: Table S1

Table S1. Values of the radius (mm) of the Fusarium sp. colonies during in vitro antagonism assays against nine Trichoderma isolates.
Author Contributions: LMM made the taxonomic identification, the in-vitro characterization of the fungal isolates, the analysis of the data, and the writing of the manuscript. AEA made a significant contribution in the manuscript's interpretation and writing. MCV designed and conducted the experiments and isolated the fungal material. All authors read and approved the final manuscript.
Funding: Not applicable
Institutional Review Board Statement: Not applicable
Informed Consent Statement: Not applicable
Data Availability Statement: All data generated or analyzed during this study are included in this published article.
Acknowledgments: Not applicable
Conflicts of Interest: The authors declare no conflict of interest
REFERENCES
1. Bartozek ECR, Lambrecht RW, Zorzal-Almeida S, Auricchio MR, Peres CK. Stream morphology, water dynamics, and agrochemicals are important drivers of periphyton biomass in subtropical streams. Hydrobiologia. 2022 Jul 1;849(13):3031–9. https://doi.org/10.1007/s10750-022-04911-y
2. Zakaria, L. (2022). Fungal and Oomycete Diseases of Minor Tropical Fruit Crops. Horticulturae, 8(4), 323. https://doi.org/10.3390/horticulturae8040323
3. Souza WCO, Nascimento LC, Oliveira MDM, Porcino MM, Silva HAO. Genetic diversity of Fusarium spp. in pineapple 'Pérola' cultivar. Eur J Plant Pathol. 2018 Apr 1;150(4):853–68. https://doi.org/10.1007/s10658-017-1328-0
4. Yamashiro, M., Arasaki, C., Takushi, et al. (2019). Fruitlet core rot of pineapple (Ananas comosus) caused by Fusarium ananatum in Japan. Nippon Shokubutsu Byori Gakkaiho, 85(1), 25-29. https://doi.org/10.3186/jjphytopath.85.25
5. Blanco-Meneses M, Castro-Zúñiga O, Umaña-Rojas G, Blanco-Meneses M, Castro-Zúñiga O, Umaña-Rojas G. Estudio preliminar de especies de Fusarium presentes en piña (ananascomosus) en Costa Rica. Agronomía Costarricense. 2022 Jun;46(1):47–64. https://doi.org/10.15517/rac.v46i1.49867
6. Zhang, X., Jin, X., Liang, X., et al. (2022). Implications of land sparing and sharing for maintaining regional ecosystem services: An empirical study from a suitable area for agricultural production in China. Sci of The Tot Env, 820, 153330. https://doi.org/10.1016/j.scitotenv.2022.153330
7. Kumar K, Thakur P, Rathore US, Kumar S, Mishra RK, Amaresan N, et al. Plant beneficial effects of Trichoderma spp. suppressing Fusarium wilt and enhancing growth in Tomato. Vegetos. 2022 Mar 1;35(1):188–95. https://doi.org/10.1007/s42535-021-00277-z
8. El-Gendy IR, El-Banobi MI, Villanueva-Jimenez JA. Bio-pesticides alternative diazinon to control peach fruit fly, Bactrocera zonata (Saunders) (Diptera: Tephritidae). Egypt J Biol Pest Control. 2021 Mar 11;31(1):49. https://doi.org/10.1186/s41938-021-00398-2
9. Mishra RK, Bohra A, Kamaal N, Kumar K, Gandhi K, GK S, et al. Utilization of biopesticides as sustainable solutions for management of pests in legume crops: achievements and prospects. Egypt J Biol Pest Control. 2018 Jan 30;28(1):3. https://doi.org/10.1186/s41938-017-0004-1
10. Cai F, Druzhinina IS. In honor of John Bissett: authoritative guidelines on molecular identification of Trichoderma. Fungal Diversity. 2021 Mar 1;107(1):1–69. https://doi.org/10.1007/s13225-020-00464-4
11. Dennis C, Webster J. Antagonistic properties of species-groups of Trichoderma: I. Production of non-volatile antibiotics. Transactions of the British Mycological Society. 1971 Jan 1;57(1):25-IN3. https://doi.org/10.1016/S0007-1536(71)80077-3
12. Dennis C, Webster J. Antagonistic properties of species-groups of Trichoderma: II. Production of volatile antibiotics. Transactions of the British Mycological Society. 1971 Jan 1;57(1):41-IN4. https://doi.org/10.1016/S0007-1536(71)80078-5
13. Prashantha A, Suryanarayana V, Patil MS, Krishnaraj, Hegde RV. Exploration of Native Trichoderma spp. from different Eco-Systems of the Canara Circle, Karnataka, India. International Journal of Environment and Climate Change. 2024 Mar 5;14(3):239–49. https://doi.org/10.9734/ijecc/2024/v14i34036
14. Askew DJ, Laing MD. An adapted selective medium for the quantitative isolation of Trichoderma species. Plant Pathology. 1993;42(5):686–90. https://doi.org/10.1111/j.1365-3059.1993.tb01553.x
15. Piontelli Laforet E. Manual de microhongos filamentosos comunes I. Escuela de Medicina, Universidad de Valparaíso. Valparaíso, Chile, 462. https://laboratoriomicologia.uv.cl/index.php?option=com_content&view=article&id=42:manualdisponible&catid=17:informaciones&Itemid=3
16. Fonseca, M. I., Zapata, P. D., Villalba, L. L., & Fariña, J. I. (2015). Characterization of the oxidative enzyme potential in wild white rot fungi from the subtropical forest of Misiones (Argentina). Acta Biolo Colomb, 20(1), 47-56. https://doi.org/10.15446/abc.v20n1.38322
17. Hammad M, Guillemette T, Alem M, Bastide F, Louanchi M. First report of three species of Trichoderma isolated from the rhizosphere in Algeria and the high antagonistic effect of Trichoderma brevicompactum to control grey mould disease of tomato. Egypt J Biol Pest Control. 2021 May 14;31(1):85. https://doi.org/10.1186/s41938-021-00423-4
18. Hall, T. (2004). BioEdit version 7.0. Distributed by the author. https://www.mbio.ncsu.edu/BioEdit/bioedit.html
19. Larsson A. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics. 2014 Nov 15;30(22):3276–8. https://doi.org/10.1093/bioinformatics/btu531
20. Sohpal VK, Dey A, Singh A. MEGA biocentric software for sequence and phylogenetic analysis: a review. International Journal of Bioinformatics Research and Applications. 2010 Jan;6(3):230–40. https://doi.org/10.1504/IJBRA.2010.034072
21. Johnson EA. An Improved Slide Culture Technique for the Study and Identification of Pathogenic Fungi. Journal of Bacteriology. 1946 Jun;51(6):689–94. https://doi.org/10.1128/jb.51.6.689-694.1946
22. Sarria G, Garcia A, Mestizo Y, Medina C, Varón F, Mesa E, et al. Antagonistic interactions between Trichoderma spp. and Phytophthora palmivora (Butler) from oil palm in Colombia. Eur J Plant Pathol. 2021 Dec 1;161(4):751–68. https://doi.org/10.1007/s10658-021-02363-z
23. Dennis C, Webster J. Antagonistic properties of species-groups of Trichoderma: III. Hyphal interaction. Transactions of the British Mycological Society. 1971 Dec 1;57(3):363-IN2. https://doi.org/10.1016/S0007-1536(71)80050-5
24. Bell, D. K., Wells, H. D., & Markham, C. R. (1982). In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathol, 72(4), 379-382. https://www.cabdirect.org/cabdirect/abstract/19821384099
25. Dal Bello, G. M. (1992). Técnica simple de bioensayo con metabolitos volátiles producidos por especies fúngicas. Revista de la Facultad de Agronomía, 68. http://sedici.unlp.edu.ar/handle/10915/120379
26. Swift ML. GraphPad Prism, Data Analysis, and Scientific Graphing. J Chem Inf Comput Sci. 1997 Mar 1;37(2):411–2. https://doi.org/10.1021/ci960402j
27. Guzmán-Guzmán P, Kumar A, de los Santos-Villalobos S, Parra-Cota FI, Orozco-Mosqueda M del C, Fadiji AE, et al. Trichoderma Species: Our Best Fungal Allies in the Biocontrol of Plant Diseases—A Review. Plants. 2023 Jan;12(3):432. https://doi.org/10.3390/plants12030432
28. Inamdar AA, Morath S, Bennett JW. Fungal Volatile Organic Compounds: More Than Just a Funky Smell? Annual Review of Microbiology. 2020 Sep 8;74(Volume 74, 2020):101–16. https://doi.org/10.1146/annurev-micro-012420-080428
29. Hamrouni R, Molinet J, Miché L, Carboué Q, Dupuy N, Masmoudi A, et al. Production of Coconut Aroma in Solid-State Cultivation: Screening and Identification of Trichoderma Strains for 6-Pentyl-Alpha-Pyrone and Conidia Production. Journal of Chemistry. 2019 Jun 9;2019:e8562384. https://doi.org/10.1155/2019/8562384
30. Vignassa M, Meile J-C, Chiroleu F, Soria C, Leneveu-Jenvrin C, Schorr-Galindo S, et al. Pineapple Mycobiome Related to Fruitlet Core Rot Occurrence and the Influence of Fungal Species Dispersion Patterns. Journal of Fungi. 2021 Mar;7(3):175. https://doi.org/10.3390/jof7030175
31. Hoyos-Carvajal L, Orduz S, Bissett J. Genetic and metabolic biodiversity of Trichoderma from Colombia and adjacent neotropic regions. Fungal Genetics and Biology. 2009 Sep 1;46(9):615–31. https://doi.org/10.1016/j.fgb.2009.04.006
32. Chinnaswami K, Mishra D, Miriyala A, Vellaichamy P, Kurubar B, Gompa J, et al. Native isolates of Trichoderma as bio-suppressants against sheath blight and stem rot pathogens of rice. Egypt J Biol Pest Control. 2021 Jan 6;31(1):12. https://doi.org/10.1186/s41938-020-00356-4
33. Kthiri Z, Jabeur MB, Machraoui M, Gargouri S, Hiba K, Hamada W. Coating seeds with Trichoderma strains promotes plant growth and enhance the systemic resistance against Fusarium crown rot in durum wheat. Egypt J Biol Pest Control. 2020 Nov 19;30(1):139. https://doi.org/10.1186/s41938-020-00338-6
34. Yang R-H, Su J-H, Shang J-J, Wu Y-Y, Li Y, Bao D-P, et al. Evaluation of the ribosomal DNA internal transcribed spacer (ITS), specifically ITS1 and ITS2, for the analysis of fungal diversity by deep sequencing. PLOS ONE. 2018 Oct 25;13(10):e0206428. https://doi.org/10.1371/journal.pone.0206428
35. Mazrou YSA, Baazeem A, Makhlouf AH, Sabry A, Ismail M, Hassan MM. Comparative molecular genetic diversity between Trichoderma spp. from Egypt and Saudi Arabia. Egypt J Biol Pest Control. 2020 Sep 29;30(1):120. https://doi.org/10.1186/s41938-020-00318-w
36. Cai F, Dou K, Wang P, Chenthamara K, Chen J, Druzhinina IS. The Current State of Trichoderma Taxonomy and Species Identification. In: Amaresan N, Sankaranarayanan A, Dwivedi MK, Druzhinina IS, editors. Advances in Trichoderma Biology for Agricultural Applications. Cham: Springer International Publishing; 2022 p. 3–35. https://doi.org/10.1007/978-3-030-91650-3_1
37. Meyer W, Irinyi L, Hoang MTV, Robert V, Garcia-Hermoso D, Desnos-Ollivier M, et al. Database establishment for the secondary fungal DNA barcode translational elongation factor 1α (TEF1α). Trends in DNA Barcoding and Metabarcoding. 2019 Jun 11;01(01):160–9. https://doi.org/10.1139/gen-2018-0083@gen-dna.issue01
38. Bissett J, Gams W, Jaklitsch W, Samuels GJ. Accepted Trichoderma names in the year 2015. IMA Fungus. 2015 Dec;6(2):263–95. https://doi.org/10.5598/imafungus.2015.06.02.02 PMID: 26734542
39. Samuels GJ, Ismaiel A, Bon M-C, De Respinis S, Petrini O. Trichoderma asperellum sensu lato consists of two cryptic species. Mycologia. 2010 Jul 1;102(4):944–66. https://doi.org/10.3852/09-243
40. Chaverri P, Branco-Rocha F, Jaklitsch W, Gazis R, Degenkolb T, Samuels GJ. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia. 2015 May 1;107(3):558–90. https://doi.org/10.3852/14-147 PMID: 25661720
41. Nofal AM, El-Rahman MA, Abdelghany TM, Abd El-Mongy M. Mycoparasitic nature of Egyptian Trichoderma isolates and their impact on suppression Fusarium wilt of tomato. Egypt J Biol Pest Control. 2021 Jul 11;31(1):103. https://doi.org/10.1186/s41938-021-00450-1
42. Abdulle YA, Osman AA, Awale MA, Heile AO, Bilal M, Subhani MN. Efficacy of Biocontrol Agents, Plant Extracts and Fungicides on Fusarium Oxysporum f. sp. Ciceris. International Journal of Plant, Animal and Environmental Sciences. 2022 Mar 31;12(1):34–43.
43. L. Knowles S, A. Raja H, D. Roberts C, H. Oberlies N. Fungal–fungal co-culture: a primer for generating chemical diversity. Natural Product Reports. 2022;39(8):1557–73. https://doi.org/10.1039/D1NP00070E
44. Promwee A, Intana W. Trichoderma asperellum (NST-009): A potential native antagonistic fungus to control Cercospora leaf spot and promote the growth of 'Green Oak' lettuce (Lactuca sativa L.) cultivated in the commercial NFT hydroponic system. Plant Protection Science. 2022 Feb 9;58. https://doi.org/10.17221/69/2021-PPS
45. Stummer BE, Zhang X, Yang H, Harvey PR. Co-inoculation of Trichoderma gamsii A5MH and Trichoderma harzianum Tr906 in wheat suppresses in planta abundance of the crown rot pathogen Fusarium pseudograminearum and impacts the rhizosphere soil fungal microbiome. Biological Control. 2022 Feb 1;165:104809. https://doi.org/10.1016/j.biocontrol.2021.104809
46. Intana W, Kheawleng S, Sunpapao A. Trichoderma asperellum T76-14 Released Volatile Organic Compounds against Postharvest Fruit Rot in Muskmelons (Cucumis melo) Caused by Fusarium incarnatum. Journal of Fungi. 2021 Jan;7(1):46. https://doi.org/10.3390/jof7010046
47. Alwadai AS, Perveen K, Alwahaibi M. The Isolation and Characterization of Antagonist Trichoderma spp. from the Soil of Abha, Saudi Arabia. Molecules. 2022 Jan;27(8):2525. https://doi.org/10.3390/molecules27082525
48. Van Poucke K, França SC, Haegeman A, Casanova E, Heungens K. Strain-specific and sensitive monitoring of the biocontrol agent Trichoderma asperellum T34 in growing medium via real-time PCR. Biocontrol Science and Technology. 2024 Apr 2;34(4):355–74. https://doi.org/10.1080/09583157.2024.2342476
49. Tian Y, Yu D, Liu N, Tang Y, Yan Z, Wu A. Confrontation assays and mycotoxin treatment reveal antagonistic activities of Trichoderma and the fate of Fusarium mycotoxins in microbial interaction. Environmental Pollution. 2020 Dec 1;267:115559. https://doi.org/10.1016/j.envpol.2020.115559
50. Tyśkiewicz R, Nowak A, Ozimek E, Jaroszuk-Ściseł J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. International Journal of Molecular Sciences. 2022 Jan;23(4):2329. https://doi.org/10.3390/ijms23042329
51. Kumar R, Samanta P, Vijay Raj S, Bera P, Naimuddin M. Potential and Prospects of Trichoderma in Plant Protection. Advances in Agriculture. 2023 Jul 31;2023:e5573662. https://doi.org/10.1155/2023/5573662
52. Choudhary AK, Singh N, Singh D. Evaluation of the bioformulation of potent native strains of Trichoderma spp. against the foot rot/gummosis of Kinnow mandarin. Egypt J Biol Pest Control. 2021 Jun 8;31(1):90. https://doi.org/10.1186/s41938-021-00437-y
53. Kannan C, Mishra D, Rekha G, Maruthi P, Shaik H, Sundaram RM. Diversity analysis of antagonistic microbes against bacterial leaf and fungal sheath blight diseases of rice. Egypt J Biol Pest Control. 2021 Aug 25;31(1):115. https://doi.org/10.1186/s41938-021-00462-x
54. Sebumpan R, Guiritan KR, Suan M, Abapo CJ, Bhat AH, Machado RAR, et al. Morphological and molecular identification of Trichoderma asperellum isolated from a dragon fruit farm in the southern Philippines and its pathogenicity against the larvae of the super worm, Zophobas morio (Fabricius, 1776) (Coleoptera: Tenebrionidae). Egypt J Biol Pest Control. 2022 Apr 25;32(1):47. https://doi.org/10.1186/s41938-022-00548-0
Received: April 20, 2023/ Accepted: May 28, 2024 / Published: June 15, 2024
Citation: Madrassi, L.M.; Alvarenga, A.E.; and Vedoya, M.C. Antagonistic activity of biocontrol agent Trichoderma spp. against Fusarium sp., the causal agent of Ananas comosus fruitlet rot. Bionatura journal 2024; 1 (2) 11. http://dx.doi.org/10.70099/BJ/2024.01.02.11
Additional information Correspondence should be addressed to lmmadrassi@hotmail.com
Peer review information. Bionatura thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Bionatura ISSN. First 13909355 Ecuador. Scopus coverage years: from 2016 to the present
Publisher's Note: Bionatura stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2024 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).