Nimotuzumab and CIMAvax-EGF® in Advanced Cervical Cancer
Raiza Ruiz
Marta Fors .2*
Marta Fors
1 Ramón González Coro Hospital. Havana, Cuba; raizaruiz@infomed.sld.cu;
1 Ramón González Coro Hospital. Havana, Cuba; daisyihd@infomed.sld.cu;
2 Center of Molecular Immunology. Havana, Cuba; carmen@cim.sld.cu;
1 Ramón González Coro Hospital. Havana, Cuba; jessicagarcia@infomed.sld.cu;
3 Universidad de Las Américas, Quito, Ecuador; forsmarthamaria@gmail.com;
2 Affiliation (Center of Molecular Immunology. Havana, Cuba);
*Correspondence: mayra@cim.sld.cu;
ABSTRACT
Cervical cancer is the fourth cancer worldwide in the female sex in terms of incidence, becoming one of the most frequent epithelial tumors. The high overexpression of the Epidermal Growth Factor Receptor (EGFr) present in it offers the opportunity to use therapies against this receptor. A prospective, multicenter Expanded Access Program (EAP) was carried out in three randomized groups to demonstrate the safety and preliminary efficacy of humanized monoclonal antibody nimotuzumab, CIMAvax-EGF® vaccine and its combination in advanced cervical cancer, refractory to all previous oncospecific therapies. The principal endpoint was to assess overall survival time (the life expectancy of these patients at the inclusion was six months) and demonstrate the safety of those treatments. Overall survival was higher than expected in all groups. In general, 43.9% of patients were alive 2 years after the start of immunotherapy, and the 60-month survival rate was 38.8, 42.7, and 37.4% for CIMAvax-EGF®, Nimotuzumab, and combination therapies, respectively.
According to overall survival, patients were separated into two groups: long (upper 24 months) and short (24 months or less) survivors. Long survivors (LS) represent 37.7% in the CIMAvax-EGF® vaccine, 49.3% in the Nimotuzumab, and 43.5% in the combination group. Adenocarcinomas (ADCs) tumors benefited from vaccine therapy, and squamous cell carcinomas with a nimotuzumab also benefited. A combination of both does not improve survival more than monotherapy. Nimotuzumab and CIMAvax-EGF® become an opportunity to treat refractory advanced cervical cancer.
Keywords: uterine cervical cancer, CIMAvax-EGF®, Nimotuzumab, Overall Survival
Cervical cancer represents the fourth most common cancer worldwide in women. In 2020, 604,127 new cases and 341,831 deaths were reported worldwide.1 The incidence in Cuba in 2017 was 1,537 cases for a crude rate of 27.2%, and mortality in 2020 corresponds to 549 cases for a crude rate of 9 .7%.2,3
Cervical uterine cancer (CUC) is one of the few preventable tumors since precursor lesions known as cervical intraepithelial neoplasia can be identified at an early stage. Developed countries, which have national screening programs, have shown a significant decrease in the incidence and mortality from this neoplasia, which is not valid in underdeveloped countries, where the absence of these leads to high incidences of cervical cancer.4
Cancer therapy is characterized by strategically integrating different treatment modalities to optimize the chances of cure. Surgery and radiotherapy are used for locoregional control of the disease. In contrast, systemic therapies (chemotherapy, endocrine therapy, and therapies based on molecular targets) are used for the control of diffuse disease (in hematological malignancies) or those that spread beyond the primary site (in solid tumors).5 Chemotherapy and radiation therapy disrupt regulatory pathways essential for tumor cell survival and growth but are limited by drug resistance mechanisms and collateral toxic damage on normal tissues.6
The oncogenic effects related to the alteration of the physiology of the Epidermal Growth Factor Receptor (EGFr) make the EGF/EGFr system a target for immunotherapeutic intervention that relies on specific antagonists of themselves.7- 9.
This overexpression of EGFr can induce the alteration of various biological functions, such as cell proliferation, migration, differentiation, and apoptosis, which lead to the transformation of a normal cell into a malignant one, promoting neo-angiogenesis and the metastasis process: signs of poor prognosis of the disease. Added resistance to radiotherapy, chemotherapy, and hormone therapy.8
Different monoclonal antibodies for therapeutic purposes can induce tumor cell death through various mechanisms: antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, and apoptosis, among others.7 The use of therapeutic vaccines against cancer helps the immune system to identify their functional or metabolic disproportion (recognize one's own), reacting to and destroying cancer cells.11
MATERIALS AND METHODS
A prospective, multicenter Expanded Access Program (EAP) was carried out in three randomized groups to demonstrate the safety and preliminary efficacy of humanized monoclonal antibody nimotuzumab, CIMAvax-EGF® vaccine and its combination in epidermoid advanced uterine cervical cancer, refractory to all previous oncospecific therapies. Patients who received the therapeutic cancer vaccine CIMAvax-EGF®, the humanized monoclonal antibody (Mab) nimotuzumab, or the combination of both were enrolled in three groups, used a random numbers list from April 4th, 2008, to May 17th, 2010. Inclusion criteria were patients with confirmed persistent, recurrent, or advanced cervical cancer from epithelial origin by cytohistological techniques, who had received all available lines of treatment for its tumor, who gave their informed consent, with an ECOG ≤ 3. Patients who were receiving another product under investigation, with decompensated chronic diseases or ECOG > 3, were excluded. The classification of adverse events was according to the standard toxicity criteria of the National Cancer Institute of United States of America (CTCAE) 3.0 version 3.0.8
Research Products
CIMAvax-EGF® vaccine: It is the result of the emulsion of the chemical conjugate of 1mg of recombinant human EGF (EGF hu-rec) P64k protein from Neisseria Meningitides (P64k rec) and montanide ISA 51 adjuvant. It was administered intramuscularly, with 4 mg of the vaccine per dose at four immunization points, two higher and two lower. The treatment was planned in two stages: induction with vaccine administration every 14 days (5 times) and maintenance every 28 days for at least two years.
Nimotuzumab (CIMAher®) is a humanized Mab, IgG1 subclass, recognizing epidermal growth factor receptor (EGFr). It was administered intravenously, 200 mg in saline solution (250 ml) per dose, over 1 hour. Treatment is divided into two stages: induction stage once a week for 12 consecutive weeks and maintenance once every 14 days for at least two years.
The combination of Nimotuzumab and CIMAvax-EGF® vaccines was administered on schedules and doses like the two previous groups on different days to identify the toxicity associated with each type of treatment.
In all cases, treatment continued beyond the progression of the disease until the appearance of unmanageable toxicity or the worsening of the patient's general condition ECOG > 3.0.
Safety. All randomized patients were evaluated after receiving at least one dose for the toxicity analysis.
Statistics. The sample size calculation used binomial distribution for pilot clinical trials of translational therapies, which allows for obtaining a value from which an upper limit of toxicity can be estimated.9 A range of patients per treatment group of 44 -49 was established by statistical criterion according to power if 80% would be used (49 patients per treatment group considering 10% additional). By presetting a maximum tolerable toxicity limit (20.0%), the sample size can be determined by considering type I error = 0.05 and a maximum tolerable toxicity ratio = 0.20. To answer the efficacy hypothesis, A bilateral hypothesis assuming type I error alpha = 0.05, type II error beta = 0.2, a difference of three months of survival related to the most frequent reference value in the literature reported in the year that the investigation begins (NCCN 2009, CRT around 6 months).10
RESULTS
The patients' age range was 22 to 77 years, with a mean of 51.1 years and 66.7% caucasian. Although only 29.4% had a relevant clinical history, comorbidities related to chronic non-communicable diseases or sequelae caused by previous oncological therapies used in them (renal failure, infections associated with fistulas, among others), this did not prevent the patients from being treated, since at the time of being evaluated for the study, they met the inclusion criteria. No significant differences were observed in the demographic variables in the groups studied. 74.4% of the patients were included with a general state (ECOG) between 0 -1, squamous cell carcinoma (SC) was the predominant histological type (54.4%). Although the characteristics of the patients in terms of the variables analyzed show a statistical balance, a significant association was observed for the histological subtypes in the three groups analyzed according to the estimation by the X2 test, with a higher proportion of endocervical adenocarcinoma (ADC) in the group treated with the CIMAvax-EGF® vaccine, with significant differences according to histological type in the three groups. (Table 1)
The patients' age range was 22 to 77 years, with a mean of 51.1 years and 66.7% caucasian. Although only 29.4% had a relevant clinical history, comorbidities related to chronic non-communicable diseases or sequelae caused by previous oncological therapies used in them (renal failure, infections associated with fistulas, among others), this did not prevent the patients from being treated, since at the time of being evaluated for the study, they met the inclusion criteria. No significant differences were observed in the demographic variables in the groups studied. 74.4% of the patients were included with a general state (ECOG) between 0 -1, squamous cell carcinoma (SC) was the predominant histological type (54.4%). Although the characteristics of the patients in terms of the variables analyzed show a statistical balance, a significant association was observed for the histological subtypes in the three groups analyzed according to the estimation by the X2 test, with a higher proportion of endocervical adenocarcinoma (ADC) in the group treated with the CIMAvax-EGF® vaccine, with significant differences according to histological type in the three groups. (Table 1)
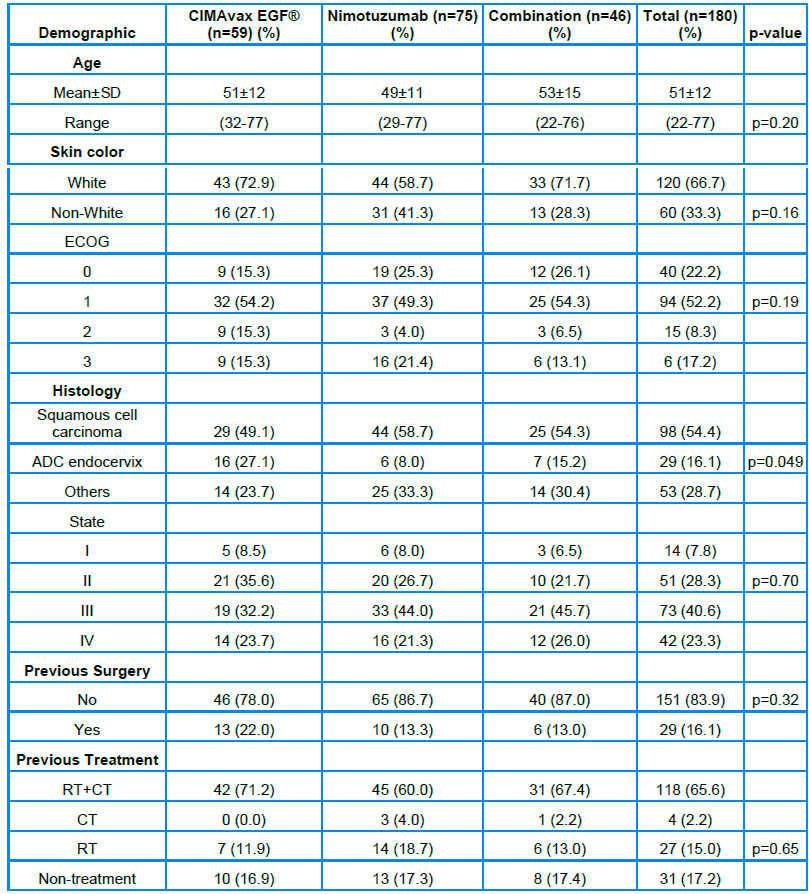
Note. SD: Standard Deviation, NA: Not Available, RT: Radiotherapy; CT: Chemotherapy
Table 1. Patients according to demographic data and treatment groups.
There is a higher proportion of long-term survivors in the groups treated with Nimotuzumab (49.3% monotherapy and 43.5% when combined with the vaccine) than in those treated with the vaccine alone. When we globally analyze the medians and survival rates, it is observed that the group that received the mAb had a superior survival; however, when dividing the patients into long and short survivors (greater or less than 24 months), it can be seen that 95.5% of the patients treated with the vaccine and who survived beyond 24 months were alive at five years, while with Nimotuzumab it was 10% less. However, the median survival rate in this group was not reached after a follow-up of 72 months. (Table 2).
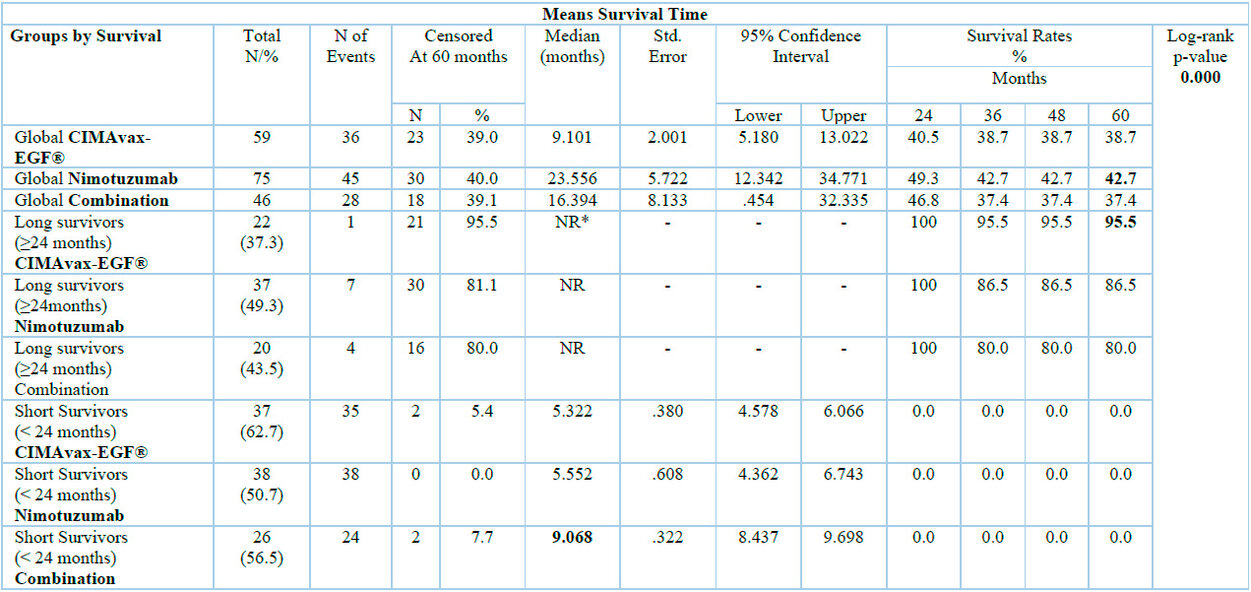
*NR: not reached
Table 2. Overall survival of the study according to global analysis, long and short survival.
Figure 1, it was represents the log-rank curves comparing the OS of the study for the three groups by ITT and separating the patients as LS or SS, a differentiation can be seen in two populations, where the patients most benefited in the LS were those who received the vaccine or the therapeutic combination as treatment, while overall the advantage for Nimotuzumab was appreciated.
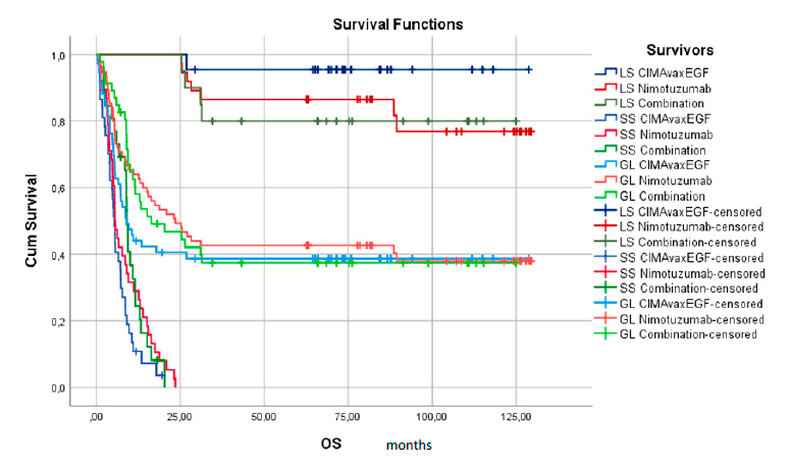
Figure 1. The survival curve for two populations according to survival time is less or more than 24 months. (LS: long Survivors, SS: Short Survivors, GL: Global Overall Survival)
For the classification of short and long survivors, the 24-month start of treatment in the three groups is taken as the reference point, observing at the time of the analysis an OS in the LS of 37.7% for the CIMAvax-EGF® vaccine, 49.3% the mAb nimotuzumab and 43.5% for the combination. Overall, 43.9% of the patients were alive 2 years after the start of immunotherapy, which, due to their clinical stage at diagnosis, had an initial life expectancy of approximately 6 months.
For the classification tree, the eight variables related to the demographic and disease characteristics of the patients included in Table 1 were analyzed, of which only the ECOG showed significance in terms of the differences in OS between the SS and LS patients. In contrast, those who presented ECOG 0 at diagnosis had an OS of 50.25 months, concerning those with the most significant involvement of the general condition.
When we consider the histology of the tumor, as shown in Table 3 and Figure 2, no significant differences are found between the two histological types when compared globally; however, the ADC treated with the combination shows a clinically superior survival than when treated with monotherapies (13.33 vs. 5.32 and 5.48 months respectively). Squamous Cell Carcinomas (epidermal carcinoma (EC)) had clinically superior survival when treated with the CIMAvax-EGF® vaccine either as monotherapy or combined with the antibody (27.07 vs 8.17 and 16.36 months respectively).
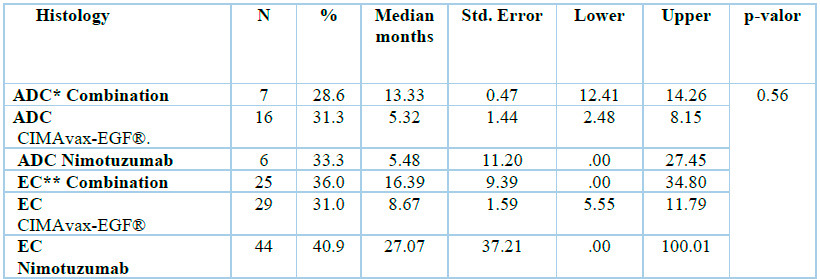
*ADC: adenocarcinoma; **EC: epidermal carcinoma
Table 3. Overall survival is related to the histology of the tumor.
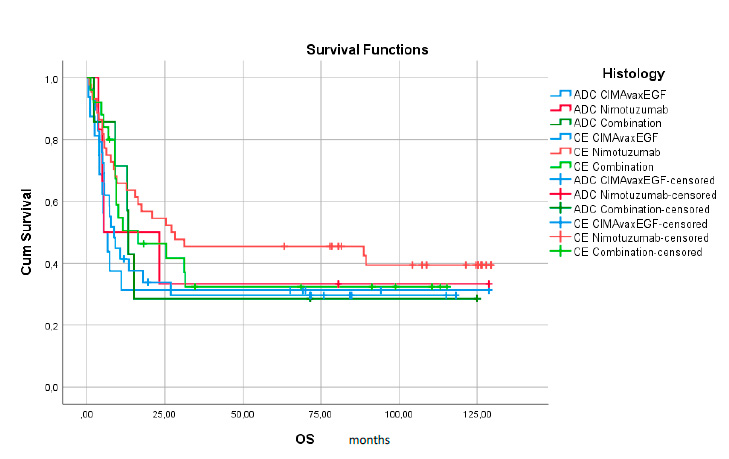
Figure 2. Survival Functions by treatment group. ADC: adenocarcinoma; CE; epidermal carcinoma; OS overall survival.
When comparing the behavior of SV in the three therapeutic groups, figure 2 shows a significant separation of the curves in the nimotuzumab group and diagnosis of squamous cell carcinoma at 27 months, although there are no statistically significant differences in clinical practice. This result is notable concerning the SV achieved with the 8.6-month vaccine and 16.3 months with the combination. It is also noteworthy to highlight these values compared to the results obtained in patients diagnosed with endocervix adenocarcinoma with an SV of only 5.48 with the mAb nimotuzumab, 5.33 with the CIMAvax-EGF® ® vaccine and 13.33 for the group of the combination.
Metastatic or refractory CUC, after chemotherapy based on platinum salts and concomitant with radiotherapy, does not have a satisfactory response in terms of disease control and survival rates worldwide, the latter being less than 15.0 % at five years, and are patients with a worse prognosis than in earlier stages12. In this context, any treatment applied to these patients aims to prolong survival, maintain or improve quality of life (QoL), and even reduce tumor growth.13
Cuba developed two products for the treatment of tumors that overexpress EGFr, based on blocking it: The Mab nimotuzumab and the therapeutic vaccine CIMAvax-EGF® (the primary activating ligand of EGFr), which are applied in this research to try to achieve an increase in survival in patients with persistent, recurrent or advanced cervical cancer.
Research in cancer therapy has focused on applying treatments that prolong the control of the disease with an adequate QoL.14, 15 Since 2014, data on long-surviving patients in the Cuban population have been reported in the indication of lung cancer. Because of the effect of the CIMAvax-EGF® vaccine and the use of complex intervention in this indication with various biotechnology medicines such as vaccines and antibodies.16
The transition from advanced cancer to cancer as a chronic disease is a concept that has recently appeared in the literature16. The characterization of the survival behavior of the treated groups by unconventional methods allows us to define the classic survival curve known as the Kaplan and Meier Curve ("with long tails") into two defined curves, where the first represents the classic population curve, and the second that of patients with prolonged survival. When this is analyzed in clinical studies that have control groups, a population also appears that, regardless of not receiving the therapy under study, presents patients with prolonged survival, and this is the one that, by incorporating a specific biological treatment such as vaccines and antibodies as in the present study, unexpectedly prolong their survival rate.16,17
The probability of being alive in this period in all treated groups is greater than 35%, a value not reported for this type of patient with advanced-stage cervical disease. No statistical differences were found between the groups, although the group treated with the mAb nimotuzumab numerically exceeds 42%. At the cut-off time (eight years), 23 (39.0%), 30 (40.0%), and 18 (39.1%) patients treated with the CIMAvax-EGF® vaccine, humanized Mab nimotuzumab, or the combination were alive (censored), which represents a group of patients with extended survival. In all groups, the results of the hypothesis were exceeded by obtaining an OS more significant than 6 months, a value reported until 2008 in patients with recurrent, persistent, or metastatic CUC treated with the chemotherapy protocols in force at that time.18
Although no significant difference was found, the humanized monoclonal antibody nimotuzumab was superior to the CIMAvax-EGF® vaccine in monotherapy and the combination with Nimotuzumab. It is recognized that the patients treated with the vaccine were the adenocarcinoma (ADC) of the endocervix (32%), with statistical significance (p = 0.049) between the treatment groups. This histological variant of cervical cancer has a poorer response to RT and CT treatments compared to tumors of epithelial origin in this location19.
In the analysis of the histological type, the results highlight a better survival in cases with a histological diagnosis of squamous cell carcinoma, which may be because ADC oncogenesis has not been determined with precision; some reports indicate that HPV 18 and 16 and their proteomes are considered responsible for oncogenesis when combined with estrogen stimulation that promotes endometrioid-type and endocervical-type proliferation.19 Patients with the ADC subtype of cervical cancer benefit from targeted agents known as MEK inhibitors, which show success in clinical trials.20 On the other hand, there are some varieties of ADC with gastric-like differentiation histology (GAS), which, although not related to HPV infections, have a worse prognosis among ADC subtypes.21
Patients with ECOG 0 had benefit when treated with the mAb nimotuzumab in short survivors (SS) and survivors (LS) patients, which is related to the concept of passive therapy since the mAb binds to the EGF receptor and inhibits cell growth, which induces the deprivation of this, and with it the inhibition of the activation of the EGFr that intervenes in the growth and tumor development. 22, 23
Although tumor histology is not revealed as a predictor of response in the dichotomous classification tree (Figure 2), it is essential to note that tumors of epithelial origin, such as squamous cell carcinomas, have a higher expression of EGF r. Since mAb nimotuzumab is a therapy explicitly directed at this receptor, it is predicted that blocking it passively inhibits the signals that it stimulates and thereby inhibits tumor growth. On the other hand, there are reports that EGF does not predominantly activate cervical uterine tumors. Schrevel et al. demonstrated that another growth factor, HB-EGF, is expressed in cervical cancer tumor cells and is the primary source of HB-EGF24. Sloss et al. also determined from the protein expression of HB-EGF, AREG, and TGFα in patients with cervical cancer that the three ligands are expressed both in the tumor stroma and the epithelial compartment. However, only HB-EGF expression is associated with EGFR expression on the tumor cell membrane, indicating HB-EGF as the primary ligand for EGFR in cervical cancer. 25 This argument allows us to explain why, in the research, the results obtained with the CIMAvax-EGF® vaccine do not have similar response indicators in terms of survival compared to the group treated with the mAb nimotuzumab or the combination of both drugs according to histological type. The operating principle of the CIMAvax-EGF® vaccine is related to the castration or reduction of circulating EGF levels, the main EGFR stimulating factor, and with this, the inhibition of its stimulation and the decrease in cell proliferation are produced, However, since the mode of stimulation is different and the HB-EGF route predominates, the reduction in EGF concentrations does not interfere with the inhibition of EGFR receptor stimulation and with this the tumor continues to grow uncontrolled, which favors the worsening of the patients, which in the long term affects overall survival.27-28.
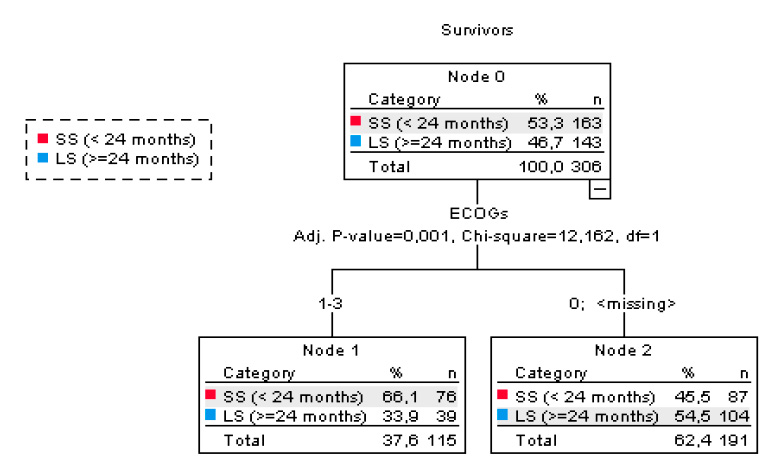
Figure 3. Classification tree by treatment groups and prognostic variables in the Expanded Clinical Use Program Dichotomic Classification tree.
Currently, the combination of chemotherapy with Bevacizumab and anti-PD-L1 antibodies, such as pembrolizumab, in patients with recurrent or metastatic CUC is a therapeutic alternative. Frenel et al. used pembrolizumab in the phase Ib KEYNOTE-028 (NCT02054806) trial. To assess safety and efficacy in PD-L1-positive advanced cervical cancer. The median progression-free Survival (PFS) was two months, and the median overall Survival (OS) was 11 months. The phase II KEYNOTE-158 (NCT02628067) study demonstrated the antitumor activity of pembrolizumab in a large cohort of patients with advanced CUC who were previously treated, being effective in patients with advanced CUC previously treated with chemotherapy.29
In the KEYNOTE-826, double-blind phase III clinical trial, where pembrolizumab was used with platinum-based CT (CDDP or carboplatin) plus Bevacizumab or not (at the investigator's discretion) every 3 weeks for 35 doses, it was obtained in 548 patients with a score of more than one (PD-L1 +) had progression-free survival of 10.4 months vs. 8.2 months in the placebo group. The overall survival rate at 24 months for the group treated with pembrolizumab was 53% and 41.7% for the placebo group, statistically significant.30 Another anti-PD-L1 mAb evaluated in the phase I/II CHECKMATE trial- 358 is nivolumab in recurrent or metastatic malignant gynecological tumors associated with HPV.31 Tisotumab (Tivdak), the first and only antibody-drug conjugate for recurrent or metastatic cervical cancer, has recently been approved by the FDA (EC-NCT 03438396). This study included 101 patients with ECOG 0 and 1, of whom 94% had extra pelvic metastatic disease, and 68% corresponded to squamous cell carcinoma. The median duration of response was 8.3 months. 32
The best median OS results were obtained in the group that was administered the mAb nimotuzumab. This result is explained by recent studies conducted by Mazorra, detailing new elements in the mechanism of action of this mAb; because it is a passive therapy that acts directly on the EGF receptors and causes inhibition of tumor proliferation, it may also have a vaccine effect since it is capable of triggering effector mechanisms of the immune system that favor death immunogenic cell, which allows control of the disease and this allows an increase in OS. The increased survival and long-term duration of responses in many patients after a short period of treatment with the mAb nimotuzumab suggests that the blockade of EGFr signaling and the resulting inhibition of tumor cell proliferation were not the unique mechanisms of action underlying the efficacy of this antibody.32,33
It is essential to point out that in the three treatment groups in this report, the five-year survival rate exceeds 30%, higher than those reported by the American Cancer Society, where the results of those patients who respond to therapies from the beginning and remain alive are exposed for long periods as a patient with prolonged survival, being these, at five years, in patients with multiple therapies and distant disease of 17%.34
CONCLUSIONS
This study demonstrates the potential of EGFr-targeted therapies in improving outcomes for patients with advanced, refractory cervical cancer. Using Nimotuzumab, CIMAvax-EGF®, and their combination resulted in significantly prolonged overall survival, with rates exceeding 35% at five years – an unprecedented finding for this patient population. These results challenge the conventional prognosis for advanced cervical cancer and suggest a potential paradigm shift towards managing the disease as a chronic condition.
Nimotuzumab emerged as a promising treatment option, demonstrating superior survival outcomes compared to CIMAvax-EGF® alone or combined. This may be attributed to its direct action on EGFr, potentially leading to a more effective blockade of tumor growth signals. While tumor histology did not definitively predict treatment response, patients with squamous cell carcinoma, known for higher EGFr expression, appeared to benefit more significantly from Nimotuzumab.
These findings highlight the need for further research into EGFr-targeted therapies for advanced cervical cancer. Future studies should focus on optimizing treatment strategies, personalizing therapy based on individual patient characteristics, and understanding resistance mechanisms to these treatments. By building on this knowledge, researchers can continue to refine treatment approaches and improve the lives of those affected by this disease.
Author contributions: Conceptualization: RR, MR, CV, MF; Data curation: RR, MR, CV; Formal analysis: RR, MR, CV, MF; Research: RR, DH, JG, MR, MF; Methodology: RR, MR, CV; Project administration: RR, MR; Material resources: RR, DH, MR, MF; Supervision: MR; Validation: MR, CV, MF; Visualization: RR, MR; Writing – original draft: RR, MR; Writing – review and editing: RR, MR, CV, MF.
REFERENCIAS
1. Hyuna Sung, Jacques Ferlay, Rebecca L. Siegel, Mathieu Laversanne, Isabelle Soerjomataram, Ahmedin Jemal, Freddie Bray. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries CA: A Cancer Journal for Clinicians. Volume 71, Issue 3May 2021Pagesi, 191-280. https://doi.org/10.3322/caac.21660 Globocan 2022. https://gco.iarc.fr/overtime/en/dataviz/bars?populations=75200_84000_19200&sexes=2&cancers=16&multiple_populations=1&types=1.
2. Ministerio de Salud Pública. Dirección de Registro Médicos y Estadísticas de Salud. Anuario estadístico de salud 2021 [Internet]. La Habana: MINSAP; 2022 [available 19/08/2022]: https://files.sld.cu/dne/files/2022/10/Anuario-Estadistico-de-Salud-2021.-Ed-2022.pdf
3. Comité de Consensos Federación Argentina de Sociedades de Ginecología y Obstetricia F.A.S.G.O. Manejo Terapéutico del Carcinoma de cuello uterino. Consenso de Ginecología 2017.http://www.fasgo.org.ar/archivos/consensos/Consenso_MANEJO_TERAPEUTICO_DEL_CARCINOMA_DE_CUELLO_UTERINO.pdf
4. Ermes L A, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res 2015 May; 3(5): 436-43. https://doi.org/10.1158/2326-6066. CIR-15-0064
5. Shen, C J, Cheng, Y M and Wang C L. lncRNA PVT1 epigenetically silences miR-195 and modulates EMT and chemoresistance in cervical cancer cells. J. Drug Target. 2017; 25: 637–644
6. Tsuchida N, Murugan A K, Grieco M. K. Ras oncogene: significance of its discovery in human cancer research. Oncotarget 2016; 7(29):46717–33. https://doi.org/10.18632/oncotarget.8773
7. Ramos-Suzarte M, Lorenzo-Luaces P, Lazo N G, Perez M L, Soriano J L, Gonzalez C E, et al. Treatment of malignant, non- resectable, epithelial origin esophageal tumours with the humanized anti-epidermal growth factor antibody nimotuzumab combined with radiation therapy and chemotherapy. Cancer Biol Ther 2012; 13(8):600-5.
8. Saurez Martínez G, Bencomo Yanes A. Nimotuzumab, effective immunotherapy for the treatment of malignant epithelial tumors. Biotecnol Apl [Internet].2014 Jun [citado 2021 Abr 08]; 31(2):159-167. http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1027 28522014000200007&lng=es
9. Saavedra D, Crombet T. CIMAvax-EGF: A New Therapeutic Vaccine for Advanced Non-Small Cell Lung Cancer Patients. Front Immunol 2017; 8: 269. https://doi.org/10.3389/fimmu. 2017.00269
10. Maen H, Terabe M, Berzofsky J A. Cancer vaccines: traslation from mice to human clinical trials. Curr Opin Immunol 2018; 51: 111-22.
https://doi.org/. 1016/j.coi. 2018.03.001
11. Cappuccio A, Castiglione F and Piccoli B. Determination of the optimal therapeutic protocols in cancer immunotherapy. MathBiosci 2007:209(1): 1-13.
12. Oaknin A and Rodriguez Freixinos V. Bevacizumab in the Treatment of Cervical Cancer Current Evidence and Next Steps. European Oncology & Haematology 2016; 12 (1):32–43.
13. Schlom J, Arlen PM and Gulley J L. Cancer vaccines: moving beyond current paradigms. Clin Cancer Res. 2007; 13(13):3776–3782.
14. Lage A. Connecting immunology research to public health: Cuban biotechnology. Nat Immunol. 2008, 9:109–112.
15. Sánchez L, Lorenzo-Luaces P, Viada C, Galan Y, Ballesteros J, Crombet T, et al.
Is there a subgroup of long-term evolution among patients with advanced lung cancer? Hints from the analysis of survival curves from cancer registry data Cancer 2014; 14: 933.http://www.biomedcentral.com/1471- 2407/14/933.
16. Lage A and Crombet T. Control of Advanced Cancer: The Road to Chronicity. Int J Environ Res Public Health 2011 (8): 683-697.
17. Mulhall, Craig J. and Coward, Jermaine I.G Targeted therapeutic management of locally advanced, recurrent, and metastatic cervical cancer. Cervical cancer: screening methods, risk factors and treatment options.Edited by Laurie Elit 2013. New York, NY United States: Nova Publishers.199-226.
18. Takeuchi S. Biology and treatment of cervical adenocarcinoma. Chin J Cancer Res 2016; 28(2): 254-262.
19. Ojesina A I, Lichtenstein L and Freeman S S. Landscape of genomic alterations in cervical carcinomas. Nature 2014; 506:371-5.
20. Kojima A, Mikami Y, Sudo T, Yamaguchi S, Kusanagiet Y, Ito M, et al. Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol 2007; 31(5):664-72.
21. Concha-Benavente F and Ferris R L. Reversing EGFR Mediated Immunoescape by Targeted Monoclonal Antibody Therapy. Front Pharmacol. 2017 May 30; 8:332. Doi: 10.3389/fphar.2017.00332. PMID: 28611673; PMCID: PMC5447743.
22. Reddy B K, Lokesh V, Vidyasagar MS, Shenoy K, Babu K G, Shenoy A, et al. Nimotuzumab provides survival benefit to patients with inoperable advanced squamous cell carcinoma of the head and neck: a randomized, open-label, phase IIb, 5-year study in Indian patients. Oral Oncol 2014 May; 50(5):498-505. Doi: 10.1016/j.oraloncology.2013.11.008. Epub 2014 Mar 6. PMID: 24613543.
23. Schrevel M, Osse M E, Prins F A, Trimbos B M Z, Fleuren G J, Gorter A, et al. Autocrine expression of the epidermal growth factor receptor ligand heparin-binding EGF-like growth factor in cervical cancer. International Journal of Oncology 50: 1947-1954, 2017, https://doi.org/10.3892/ijo.2017.3980
24. Sloss C M, Wang F, Palladino M A and Cusack J C. Activation of EGFR by proteasome inhibition requires HB‐EGF in pancreatic cancer cells. Oncogene 2010; 29: 3146-3152. 265. Kato T, Takashima A, Kasats T, et al. Clinical tumor diameyer and prognosis of patients whit FIGO satge IB1 cervical cancer (JCOGO806-A). Gynecol Oncol 2015; 137: 34-39. http://www.ncbi.nlm.nih.gov/pudmed/25662625.
25. Concha-Benavente F and Ferris R L. Reversing EGFR Mediated Immunoescape by Targeted Monoclonal Antibody Therapy. Front Pharmacol. 2017 May 30; 8:332. Doi: 10.3389/fphar.2017.00332. PMID: 28611673; PMCID: PMC5447743.
26. González G, Crombet T, Catalá M, Mirabal V, Hernández J C, González Y, et al. A novel cancer vaccine composed of human-recombinant epidermal growth factor linked to a carrier protein: report of a pilot clinical trial. Ann Oncol. 1998; 9 (4):431–5.
27. Rodríguez P C, Neninger E, García B, Popa X, Viada C, Luaces P, et al. Safety, immunogenicity, and preliminary efficacy of multiple-site vaccination with an Epidermal Growth Factor (EGF) based cancer vaccine in advanced non-small cell lung cancer (NSCLC) patients. J Immune Based Ther Vaccines 2011 Oct 24; 9:7. Doi: 10.1186/1476-8518-9-7. PMID: 22024351; PMCID: PMC3215653.
28. Chung H C, Ros W, Delord J P, Perets R, Italiano A, et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results from the Phase II KEYNOTE-158 Study. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2019 Jun; 37(17):1470-1478. https://doi.org/10.1200/jco.18.01265
29. Colombo N, Dubot C, Lorusso D, Caceres M V, Hasegawa K, Shapira‐Frommer R, et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer.The New England Journal of Medicine. 18, 2021, at NEJM.org. https://doi.org/10.1056/NEJMoa2112435
30. Haddad R, Blumenschein G Jr, Fayette J, Guigay J, Colevas A D, Licitra L, et al. Treatment Beyond Progression with Nivolumab in Patients with Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck in the Phase 3 Checkmate 141 Study: A Biomarker Analysis and Updated Clinical Outcomes. Annals Oncol 2017; 28 (5): 372-394.
31. Mazorra Z, Lavastida A, Concha-Benavente F, Valdés A, Srivastava R M, García-Bates T M ,et al. Nimotuzumab Induces NK Cell Activation, Cytotoxicity, Dendritic Cell Maturation and Expansion of EGFR-Specific T Cells in Head and Neck Cancer Patients. Frontiers in pharmacology. 2017; 8: 382.
32. Mazorra H Z and Crombet R T. Pilot study of a novel combination of two therapeutic vaccines in advanced non‑small‑cell lung cancer patients. Cancer Immunol Immunother. 2014; 63:737–747. https://doi.org/10.1007/s00262-014-1552-9
33. NCCN. Clinical Practice Guidelines in Oncology. Version 1.2023, 01/06/23 © 2023 National Comprehensive Cancer Network®. https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
Received: September 30, 2024 / Accepted: November 13, 2024 / Published: December 15, 2024
Citation: Ruiz R , Hernández D, Viada C , García J, Fors M , Ramos M. Nimotuzumab and CIMAvax-EGF® in Advanced Cervical Cancer. Bionatura journal. 2024;1(4):20. doi: 10.70099/BJ/2024.01.04.20
Additional information Correspondence should be addressed to mayra@cim.sld.cu
Peer review information. Bionatura thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
ISSN.3020-7886
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Publisher's Note: Bionatura Journal stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2024 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




