Biodegradation efficiencies of Low Pour Fuel Oil by Pseudomonas aeruginosa and Bacillus licheniformis isolates
Chinedu Emeka Ihejirika1 Joseph Ifeanyichukwu Garricks1, Ejeagba Okorie Imo2*, Joseph Ikechukwu Nwachukwu1, Ihuoma Ezichi Mbuka-Nwosu1, Etienne Chukwuma Chinakwe2, Ursula Ngozi Nwaogwugwu2,Christopher Chibuzor Ejiogu1, Obenade Moses3
1Department of Environmental Management, Federal University of Technology, Owerri, Nigeria
2Department of Microbiology, Federal University of Technology, Owerri, Nigeria
3Department of Geography and Environmental Management, Federal University, Ndiofe Alaike Ebonyi State, Nigeria
*Correspondence: ejeagba.imo@futo.edu.ng
ABSTRACT
This study explored the efficiencies of Pseudomonas aeruginosa and Bacillus licheniformis isolates in the degradation of a derivative of crude oil, Low Pour Fuel Oil (LPFO), commonly known as Black oil. The comparison was carried out on the effects of nutrient stimulation on the degradation of LPFO by the selected organisms. After a 14-day treatment, correlational analysis of the biodegradation test showed a significant solid correlation between organisms and different treatments at p<0.01. There was an increase in the counts of B. licheniformis and P. aeruginosa during the degradation process. The susceptibility of the hydrocarbon compounds to microbial degradation varied with the type and size of the hydrocarbon molecules. Alkanes of intermediate chain length (C10–C24) were degraded rapidly compared to long-chain alkanes (C20–C34). There was a significant increase in degradation when the LPFO was inoculated with B. licheniformis and P. aeruginosa, while there was no significant effect of nutrient amendment on the hydrocarbon degradation compared to treatments with individual microorganisms alone. The average Degradation Efficiency was 99.9%. Pseudomonas aeruginosa and Bacillus licheniformis isolates were influential in the degradation of LPFO and can be employed in the remediation of contaminated soil.
Keywords: biodegradation, bio-stimulation, biotechnology, hydrocarbon, low-pour fuel oil
INTRODUCTION
Increasing industrialization associated with economic growth and demand for more new derivatives of crude oil and consequent spillages have presented more challenges to applications
of biotechnology in the remediation of hydrocarbon-contaminated environment1. Varying chemical compositions and structures of these different new derivatives of hydrocarbons may affect bioavailability2, biodegradability3, and remediation efficiencies4 of microbes. It may further pose challenges to selecting consortia of microbes for remediation, considering environmental factors, and resulting in microbial growth inhibition. 5,1
The Low Pour Fuel Oils (LPFOs), called Black oil, are products derived from crude oil with major components residuals of petroleum refining operations6, 7. LPFOs, also known as Light oil, Marine oil, or Furnace oil, are a fraction obtained from petroleum distillation, either as a distillate or residue. LPFOs are fundamental input in steam generation in many labor-intensive industries like textiles (coloring), construction (cement), food (sugar), and beverages (sterilizing). Residual or distillate oil is one of the lowest-value petroleum products from a refinery. It is a by-product of light products like Premium Motor Spirit or Regular Motor Spirit (Petrol), Dual Purpose Kero or Household Kero (Kerosene), and Naphthalene 6,7. LPFO is blended with other petroleum products to meet customer specifications6.
According to Ohijeagbon et al., increased density of emissions from boiler operations of LPFOs would lead to increasing greenhouse effects and global warming by releasing such greenhouse gases as carbon dioxide, nitrous oxide, and water vapour8. Studies have shown that the shear strength of different soil types is reduced with increasing soil contamination with LPFO9,7. According to Otunyo and Anele, more damaging effects on the soil properties caused by LPFO spillage included an increase in consolidation settlement for used engine oil-contaminated laterite, which, in the case of clay soil, generally decreases with all the contaminants 9. Effects of spillages of crude oil derivatives have silently constituted a major environmental concern, especially outside the areas of oil exploration10. The spillage may result from leakage of pipes, disruption of storage tanks, and failures in processing plants, refineries, and transport systems 10. These derivatives may be discharged in large pools or areas easily dispersed by other environmental events. These derivatives may, through penetration, reach more profound levels of the earth's crust, constituting remediation challenges, especially in agricultural lands.11 The increasing usage of most derivatives of crude oil like LPFOs and its by-products may be responsible for the uncontrolled dispersal of unused portions, spent oils, and their mixture in the environment.
Spillages occur during the production process, and environmental damages are evidently observed on vegetation and soil12. There might also be surface and groundwater contamination through rainwater runoffs on the surfaces of contaminated soils.13, 14
Restoration of the polluted soil can be attributed to employing bioremediation methods.15,16,17 This can be achieved through the informed introduction of microbial agents and nutrient enhancement to optimize bioremediation of the contaminated soil18. According to Xu et al., the reduction of chemical compounds by biological catalysis is known as biodegradation. 19 Furthermore, Xue et al. defined microbial degradation of organic compounds into minute units as biodegradation. 20 Invariably, biodegradation is a process of mineralization of complex compounds. The rate of biodegradation has been established to depend on essential factors like biotic, concentration of contaminant or substrate19, the amount of "catalyst" or microbial population21, environmental and abiotic factors22, and genetic or species of microbes19. Biodegradation has been employed extensively to remove environmental contaminants23, especially those classified as intermediate refinery products that pose significant challenges.
These may be everyday experiences at spill sites of LPFO at Okari Jetty, a unit at Port Harcourt Refinery, Rivers State, and other oil-producing areas of Niger Delta, South-southern Nigeria. This study, therefore, explored the efficiencies of Pseudomonas aeruginosa and Bacillus licheniformis isolates in the degradation of LPFO in a laboratory test experiment. Biostimulation experiments were also carried out to compare the effectiveness of nutrient addition to laboratory treatments in the degradation of LPFO by Pseudomonas aeruginosa and Bacillus licheniformis isolates. These will further enhance the cleanup and remediation efforts of LPFOs' contaminated environment and prevent environmental consequences of LPFOs' pollution.
MATERIAL AND METHODS
Sample collection
Triplicate samples of Low Pour Fuel Oil were collected with the aid of sterilized 1 Litre amber glass bottles, with Teflon-lined lids below the point source of the discharge at the spill sites at Okari Jetty, a unit at Port Harcourt Refinery, Rivers State, Nigeria. The sample bottles were securely closed and packed carefully to prevent leaking or breakage, labeled, and immediately transported to the laboratory for analysis.
A composite mixture of fifty grams (50g) of materials from the gastrointestinal contents of five slaughtered cows was collected with sterile transport containers at an abattoir in Owerri, Imo State, Nigeria. Drops of sterile saline were added to keep small pieces of materials moist. The samples were then transported to the microbiology laboratory. At the laboratory, one gram (1g) of the gastrointestinal materials was transferred into a 9ml test tube of sterilized distilled water and subjected to a 10-fold serial dilution and bacterial isolation and characterization.
Media Preparation
Preparation of Pseudomonas P agar
Pseudomonas P agar (Sigma-Aldrich, Darmstadt, Germany) was prepared per the manufacturer's directives. A 46.4 g medium was suspended in 1000 ml of demineralized water containing 10 ml of glycerol. To completely dissolve the suspension, it was heated to boiling with agitation. The solution was sterilized by autoclaving at 15 lbs pressure at 121°C for 15 minutes. The sterilized solution was dispensed into Petri dishes to cool at room temperature.
Preparation of Bushnell-Haas broth
Bushnell-Haas broth was prepared according to the method outlined by Bushnell and Haas24 (1941). A 3.27g Bushnell Haas broth (HiMEDIA, USA) was suspended in 1000 ml of distilled water. The suspension was boiled for 1 minute to dissolve the medium completely. The mixture was sterilized by autoclaving at 15 lbs. pressure at 121°C for 15 minutes.
Isolation and identification of Bacillus licheniformis
Isolation and identification of Bacillus licheniformis from samples of gastrointestinal materials of cows was performed according to the method by Wang and Shih25. Wang and Shih25 composed Basic Growth Medium with a Minimum Growth Medium (MGM) made of (in gl-1): NaCl, 0.5; KH2PO4, 0.7; K2HPO4, 1.4 and MgSO4.7H2O, 0.1 at pH 7. Further identification of Bacillus licheniformis was carried out through biochemical characterization as described by Al-Dhabaan26.
Plate count and enumeration
Triplicate plates of each dilution were used to achieve a plate count of between 30 – 300 colonies forming units/ml (cfu/ml) as described by Prescott27.
Colonial and Microscopic Characteristics of Test Cultures
Gram staining
The Gram Staining method of Cruickshank28 was adopted to determine the Gram staining reactions of all bacterial isolates. With the help of a sterile wire loop, individual isolate was smeared onto clean microscope slides, air-dried, and fixed. Crystal violet solution was poured on the smear and, after 30 seconds, washed in gentle running water for 5 seconds, covered with iodine solution, and washed again. The stain-smear was then decolorized using 95% ethanol, washed with water, and finally counter-stained with 3% Safranin solution for 15 seconds. The smear was washed in clean tap water, air–dried, and observed first under X60 magnification and finally under an oil immersion object lens (X100).
P. aeruginosa was isolated and identified with the aid of the Cetrimide Agar medium, which is selective for P. aeruginosa and other species of Pseudomonas. Cetrimide Agar medium is made up of Cetrimide, which is known for inhibiting the growth of numerous bacteria, including gram-positive bacteria, while Pseudomonas species, especially Pseudomonas aeruginosa, proliferate.Biochemical characterization of bacterial isolates
Further identification was carried out through biochemical characterization as follows.
Nitrate reduction test
Test tubes with nitrate broth and inverted Durham's tube were autoclaved. These were allowed to cool at room temperature. The bacteria isolates from fresh (24 hours old) cultures were then inoculated into the tubes using a sterile inoculating loop. The tubes were thereafter incubated at 37ºC for 24 hrs. The presence of gas in the Durham's tube showed a positive result.
Methyl red (MR) and Voges Proskaur (VP) test
After adding methyl red indicator solution (TSBA, Himedia) to inoculated culturing media and incubation at 35 °C for up to 4 days, the change of color to red indicates a positive MR result 29.
Oxidase test
This test was carried out by smearing a filter paper earlier saturated with freshly prepared oxidase reagent with bacterial colony. A positive oxidase test was recorded as the development of a blue-purple color within 10 s 30.
Test for fermentation of sugars
Bacterial isolates were tested for their ability to ferment sugars like maltose, lactose, glucose, and sucrose. The medium fermentor was thoroughly mixed, and 9ml was dispensed into each clean, dry test tube containing Durham tubes and autoclaved at 1210C for 15 minutes. A 10% stock solution of each of the sugars to be tested was prepared and sterilized by autoclaving at 1150C for 10 minutes. One milliliter of sterile 10% sugar solution was put in each fermentation medium. A 0.1ml isolated organism emulsified in sterile peptone water was used to inoculate such a tube and incubated at 370C for 24 hours. Positive sugar fermentation was indicated by a change from purple to the yellow color of the medium due to acid production accompanying sugar fermentation. In tubes where fermentation was accompanied by gas production, such gas will accumulate in the inverted Durham tubes.
Catalase test
A catalase test was carried out by adding a purified bacterial culture to 5 ml hydrogen peroxide solution. Detection of gas bubbles within 10 seconds after adding bacterial culture indicated a positive catalase test30.
Coagulase test
A tube containing ½ ml rabbit plasma was inoculated with the bacterial inoculum. This was incubated at 37º C and checked at ½ hour or the next lab period by tipping the slide at an angle. The absence of coagulation is considered a negative test for the lack of coagulase enzyme.
Urease test
Bacterial colonies were added to slanted bijou bottles filled with two millilitres of urea medium and incubated at room temperature. Red-pink color in the medium was considered as a positive test for urease induction30.
Indole test
Bacterial colony added to SIM media was applied with 0.5 ml of Kovac's reagent and incubated at 35 °C for 24 h. The appearance of bright red and yellow colors indicated positive and negative results, respectively30.
Simmons Citrate test
Simmons Citrate test was performed via inculcating Simmons Citrate Agar plates (TSBA, Himedia) surface with bacterial cultures, then incubated at 37 °C up to 48 h. changing the media color from green to bright blue indicates a positive reaction.
Methyl red (MR) test
After adding methyl red indicator solution (TSBA, Himedia) to inoculated culturing media and incubating at 35 °C for up to 4 days, changing color to red indicates MR test positive- the appearance of tested bacteria29.
Gelatin hydrolysis
The nutrient gelatin stab method was applied according to Edison31. Heavy inoculums of a test bacterium inoculated into tubes containing nutrient gelatin, gelatin liquefaction is the positive result for bacterial gelatin hydrolysis.
Serial Dilution of Low-Pour Fuel Oil
Nine milliliters (9 ml) of sterile distilled water (without nutrients) and 9 ml of sterile Bushnell Haas medium (with nutrients) were prepared in duplicates, and the test tubes were covered with sterile cotton wool to prevent contamination.
Analysis of hydrocarbon concentrations
Reagents: HPLC grade hexane (Spectrum Chemical, USA), HPLC grade Dichloromethane (Sigma-Aldrich, China), Analytical grade Acetone (Alliance Chemical, Texas USA), Anhydrous Sodium tetra-oxosulphate (vi) (Na2SO4) (Sigma-Aldrich, Germany), Chromic Acid (Sigma-Aldrich, Germany), Glass wool (Sigma-Aldrich, Germany) and Silica Gel (Sigma-Aldrich, Germany).
Apparatus/Equipment
The equipment used was HP 5890 Gas Chromatography equipped with Chem Station software workstation, capillary column, Flame Ionization Detector (FID) and chemstation software workstation, rotary evaporator, vials for storing extracts (2ml), and separatory funnel.
Procedure:
A 1:1 solvent mix of acetone/ Dichloromethane (DCM) was prepared, and 10 ml of low-paste fuel oil sample was added to an acid-washed acetone beaker devoid of water. Fifty milliliters (50ml) of Acetone/DCM solvent mix was added into the beaker. The sample was then placed in a sonicator and sonicated for 15 minutes at 65°C and was centrifuged for 10 minutes at 55°C. Samples were then carefully extracted by adding 5g of anhydrous sodium sulfate, and the samples were concentrated into 2 ml using a rotary evaporator. Components of samples were then fractionated into Aliphatic and Aromatic hydrocarbons using column chromatography packed with glass wool and silica gel; the packed column was then preconditioned with hexane through the procedure of Gas Chromatography-Mass Spectrometry (GC-MS) 32.
Test for pH
Each treatment sample was immersed with a clean, sterile electrode at a sufficient depth to allow immersion. The treatment samples were mixed by shaking and stirring for a few minutes and allowed to stand for 15 minutes. After a steady reading of the meter, the pH of the treatment was recorded.
Test for Electrical Conductivity (µS/cm)
The Electrical Conductivity (EC) meter electrode was immersed into the slurry, and the needle drift was waited to cease. The EC value was recorded for each sample.
Biodegradation treatments
Duplicate test tubes of 9 ml of sterile distilled water (without nutrients) and 9ml of sterile Bushnell Haas medium (with nutrients) broth were prepared in duplicates, as recommended by the Society for Industrial Microbiology (SIM) Committee on Microbiological Deterioration of Fuels33. A 0.1ml Bacillus licheniformis and Pseudomonas aeruginosa inocula were inoculated directly into the Bushnell Haas broth in duplicate test tubes. The Bushnell Haas broth was then overlaid with 1ml of sterile LPFO. These were also repeated with sterilized distilled water (without nutrients), devoid of Bushnell-Haas broth as the nutrient, to compare the effects of nutrient stimulation in the degradation of LPFO. These inoculated broths were incubated aerobically at 25-30°C for two weeks (14 days). The tubes were examined daily for growth by growth count and recorded every two days.
Initial values of total hydrocarbon concentration before inoculation with Bacillus licheniformis and Pseudomonas aeruginosa inocula and after incubation for 14 days were determined and recorded according to the different experiments.
Computation of degradation efficiency
Degradation efficiency of bacterial isolates was calculated using the following equation:
DE = (Ic - Fc)/Ic x 100% ………………………………………………………….Equa. (1)
Where, DE = Degradation efficiency (%)
Fc = Final concentration of hydrocarbon after treatment
Ic = Initial concentration of hydrocarbon before treatment
Statistical analysis
The results were subjected to descriptive statistics, Analysis of Variance, Duncan Multiple analysis, and correlation.
RESULTS
Bioremediation Treatments
In Table 1, the pH values of the biodegradation experiments after degradation were slightly acidic; degradation with P. aeruginosa (without nutrients, P) at pH 6.65±0.01; with P. aeruginosa (with nutrients, Po) at pH 6.41±0.01; with B. licheniformis (without nutrients) at pH 5.87±0.01; and with B. licheniformis (with nutrients, Po) at pH 5.95±0.01. The pH varied significantly from all the treatments at p <0.05.
The Electrical Conductivity (EC) of the biodegradation experiments was thus: with degradation with P. aeruginosa (without nutrients, P) at 112.3±0.1 µS/cm; P. aeruginosa (with nutrients, Po) at 108.6±0.10 µS/cm; degradation with B. licheniformis (without nutrients) at 134.4±0.10 µS/cm;
and B. licheniformis (with nutrients, Po) at 129.8±0.10 µS/cm. The EC varied significantly from all the treatments at p <0.05.
The Total Petroleum Hydrocarbon (TPH) concentrations in the initial concentration of LPFO (35560mg/L) were after 14 days thus, with B. licheniformis (without nutrients) to
2.3682±.0001 mg/L; with P. aeruginosa (without nutrients, P) to 9.9021±.0001 mg/L; with B. licheniformis (with nutrients, Po) to 2.878±.0001 mg/L and with P. aeruginosa (with nutrients, Po) to 9.9879±.0001 mg/L. The degradation efficiencies were thus: degradation with P. aeruginosa (without nutrients, P) at 99.97%; P. aeruginosa (with nutrients, Po) at 99.97%; degradation with B. licheniformis (without nutrients) at 99.99%; and B. licheniformis (with nutrients, Po) at 99.99%, with an overall average treatment of 99.9% efficiency.
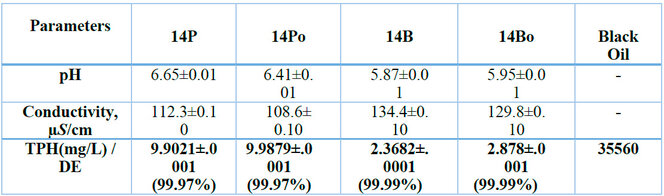
Legends: P = P. aeruginosa only, Po = P. aeruginosa with nutrient, B = B. licheniformis only, Bo = B. licheniformis with nutrient, TPH = Total petroleum hydrocarbon, DE = Degradation Efficiency
Table 1: Final levels of the qualities of treatment and Degradation Efficiencies
Table 1: Final levels of the qualities of treatment and Degradation Efficiencies
The chromatographs in Fig. 1 were used as the control, which showed the initial concentrations of the petroleum hydrocarbon compounds, with the peaks of individual components of carbon chains, before biodegradation of LPFO with any of the bacterial isolates. Fig. 2 shows the levels of the final concentration of the petroleum hydrocarbon compounds, with the peaks of individual components of carbon chains, Bacillus licheniformis (without nutrients), after 14 days of biodegradation of LPFO. Fig. 3 shows the levels of the final concentration of the petroleum hydrocarbon compounds, with the peaks of individual components of carbon chains, Pseudomonas aeruginosa (without nutrients), after 14 days of biodegradation of LPFO. Fig. 4 shows the levels of the final concentration of the petroleum hydrocarbon compounds, with the peaks of individual components of carbon chains, Bacillus licheniformis (nutrients), after 14 days of biodegradation of LPFO.
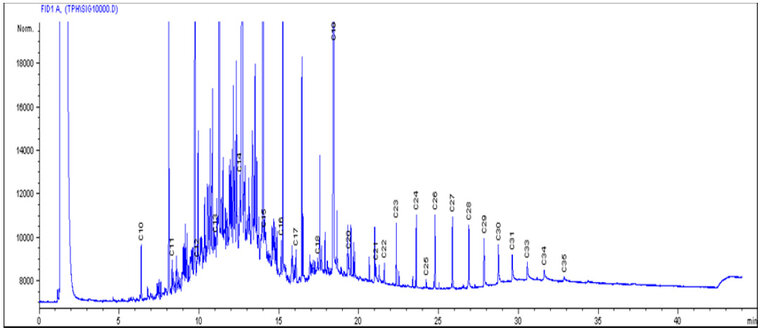
Figure 1: Initial values of LPFO in the sample before degradation
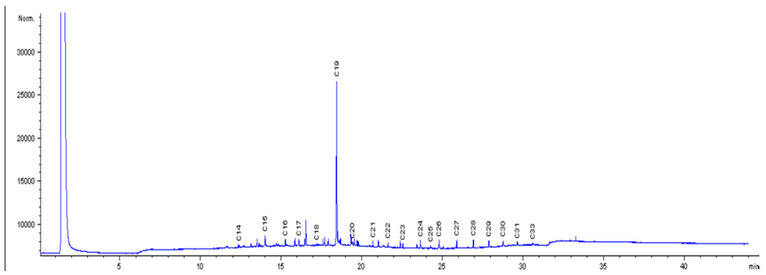
Figure 2: Degradation of LPFO by Bacillus licheniformis without addition of nutrient
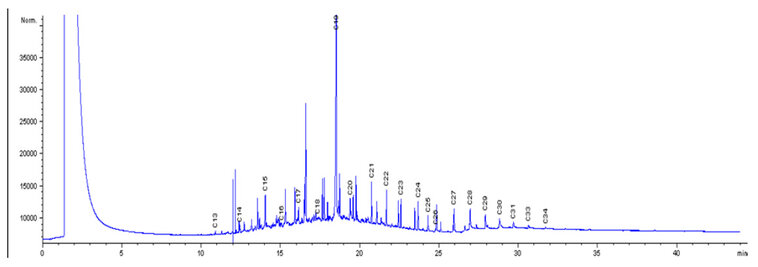
Figure 3: Degradation of LPFO with B. lichineformis with nutrient
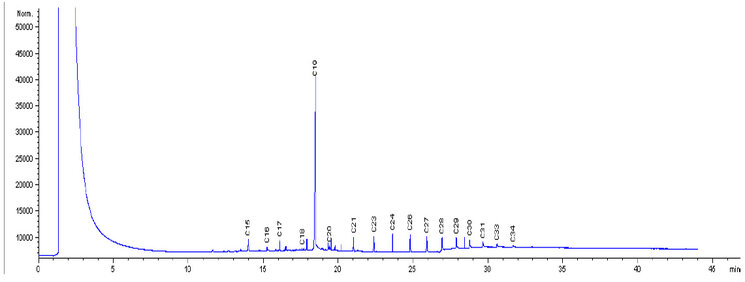
Figure 4: Degradation of LPFO by Pseudomonas aeruginosa with nutrient
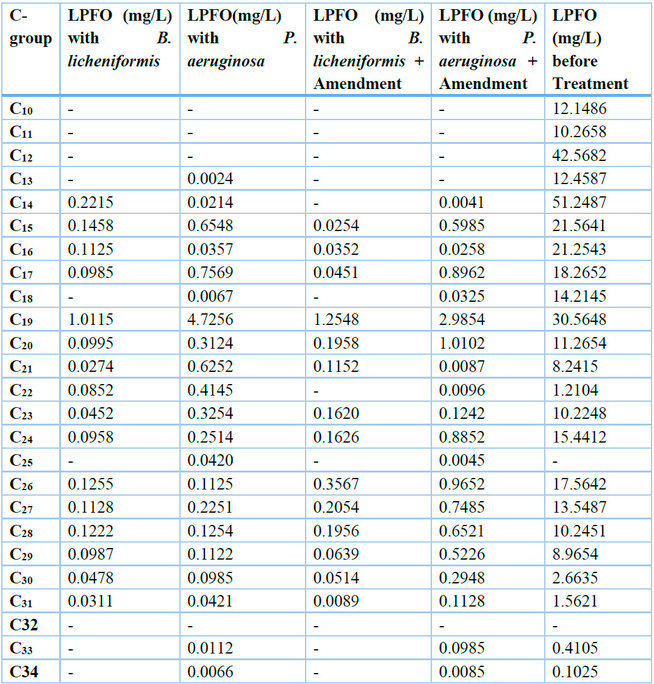
Table 2: Hydrocarbon Concentrations after 14 days Different Treatments
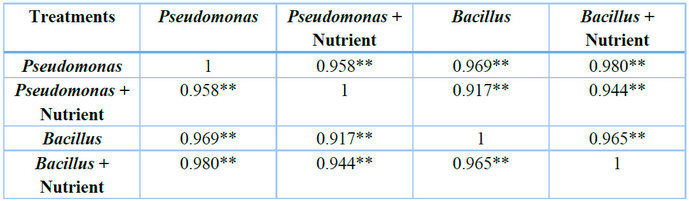
** = Significant at p < 0.01
Table 3: Correlation of different efficiencies of treatments in the biodegradation of LPFO
In Table 2, the initial concentration of LPFO (35560mg/L), after 14 days of treatment, individual carbon compounds were reduced thus: with Bacillus licheniformis (without nutrients), C14(51.2487 to 0.2215mg/L), C15(21.5641 to 0.1458mg/L), C16(21.2543 to 0.1125mg/L), C17(18.2652 to 0.0985mg/L), C18(14.2145 to 0mg/L), C19(30.5648 to 1.0115mg/L), C20(11.2654 to 0.0995mg/L), C21(8.2415 to 0.0274mg/L), C22(1.2104 to 0.0852mg/L), C23(10.2248 to 0.0452mg/L), C24(15.4412 to 0.0958mg/L), C26(17.5642 to 0.1255mg/L), C27(13.5487 to 0.1128mg/L), C28(10.2451 to 0.1222mg/L), C29(8.9654 to 0.0987mg/L),C30(2.6635 to 0.0478mgL), C31(1.5621 to 0.0311mg/L), C33(0.4105 to 0mg/L) and C34(0.1025 to 0mg/L); with Pseudomonas aeruginosa (without nutrients) reduced to C13(12.4587 to 0.0024mg/L), C14(51.2487 to 0.0214mg/L), C15(21.5641 to 0.6548mg/L), C16(21.2543 to 0.0357mg/L), C17(18.2652 to 0.7569mg/L), C18(14.2145 to 0.0067mg/L), C19(30.5648 to 4.7256mg/L), C20(11.2654 to 0.3124mg/L), C21(8.2415 to 0.6252mg/L), C22(1.2104 to 0.4145mg/L), C23(10.2248 to 0.3254mg/L), C24(15.4412 to 0.2514mg/L), C25(0 to 0.0420mg/L), C26(17.5642 to 0.1125mg/L), C27(13.5487 to 0.2251mg/L), C28(10.2451 to 0.1254mg/L), C29(8.9654 to 0.1122mg/L), C30(2.6635 to 0.0985mgL), C31(1.5621 to 0.0421mg/L), C33(0.4105 to 0.0112mg/L) and C34(0.1025 to 0.0066mg/L); with Bacillus licheniformis (nutrients) reduced to C14(51.2487 to 0mg/L), C15(21.5641 to 0.0254mg/L), C16(21.2543 to 0.0352mg/L), C17(18.2652 to 0.0451mg/L), C18(14.2145 to 0mg/L), C19(30.5648 to 1.2548mg/L), C20(11.2654 to 0.1958mg/L), C21(8.2415 to 0.1152mg/L), C22(1.2104 to 0mg/L), C23(10.2248 to 0.1620mg/L), C24(15.4412 to 0.1626mg/L), C26(17.5642 to 0.3567mg/L), C27(13.5487 to 0.2054mg/L), C28(10.2451 to 0.1956mg/L), C29(8.9654 to 0.0639mg/L),C30(2.6635 to 0.0514mgL), C31(1.5621 to 0.0089mg/L), C33(0.4105 to 0mg/L) and C34(0.1025 to 0mg/L); and with Pseudomonas aeruginosa (nutrients) reduced to C14(51.2487 to 0.0041mg/L), C15(21.5641 to 0.5985mg/L), C16(21.2543 to 0.0258mg/L), C17(18.2652 to 0.8962mg/L), C18(14.2145 to 0.0325mg/L), C19(30.5648 to 2.9854mg/L), C20(11.2654 to 1.0102mg/L), C21(8.2415 to 0.0087mg/L), C22(1.2104 to 0.0096mg/L), C23(10.2248 to 0.1242mg/L), C24(15.4412 to 0.8852mg/L), C26(17.5642 to 0.9652mg/L), C27(13.5487 to 0.7485mg/L), C28(10.2451 to 0.6521mg/L), C29(8.9654 to 0.5226mg/L),C30(2.6635 to 0.2948mg/L), C31(1.5621 to 0.1128mg/L), C33(0.4105 to 0.0985mg/L) and C34(0.1025 to 0.0085mg/L).
Variations in microbial counts of different samples subjected to treatment
Microbial counts of the samples subjected to treatments with Pseudomonas (1.21 x 109 ± 2.07 x 109cfu/ml) and Pseudomonas with nutrient (1.3 x 109 ± 1.66 x 109cfu/ml) did not vary significantly at p > 0.108; Pseudomonas and Bacillus (4.5 x 108 ± 5.86 x 108cfu/ml) varied significantly at p < 0.001; Pseudomonas and Bacillus with nutrient (3.51 x 109 ± 5. 585 x 109cfu/ml) did not vary considerably at p > 0.184; Pseudomonas with nutrient and Bacillus varied significantly at p < 0.044; Pseudomonas with nutrient and Bacillus with nutrient varied significantly at p < 0.00; while Bacillus and Bacillus with nutrient did not differ considerably at p > 0.069.
Table 3 below compares the initial concentrations of LPFO and their final concentrations after 14 days of treatment. It was observed after 14 days that the concentration of the petroleum hydrocarbon (PH) compounds reduced significantly when treated with microbial cultures and the addition of nutrients. There was a significant reduction in the concentration of the compound C19 when treated with B. licheniformis and B. licheniformis + nutrient compared with the concentration of LPFO before the samples were subjected to treatment. This same trend was observed in most of the component carbon compounds of the LPFO.
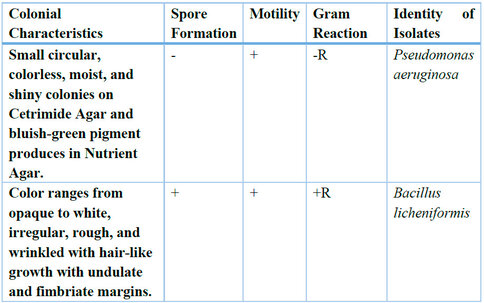
Legends: R= rod-shaped; + = positive test; − = negative test
Table 4: Colonial and Microscopic Characteristics of Test Cultures
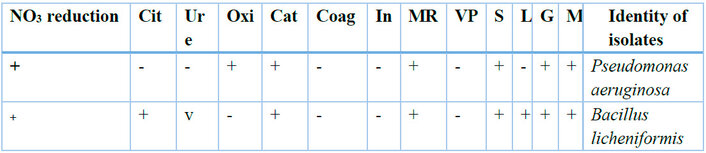
Legends: NO3 = nitrate reduction test; Cit = citrate utilization test; Ure = Urease; Oxi = oxidase test; Cat = Catalase test; Coag = coagulase test; In = indole test; MR = Methyl Red test; VP = Voges Proskaeur test; S = sucrose; L = lactose; G = glucose; M = maltose; v = variable
Table 5: Microscopic and Biochemical Characteristics of Test Cultures
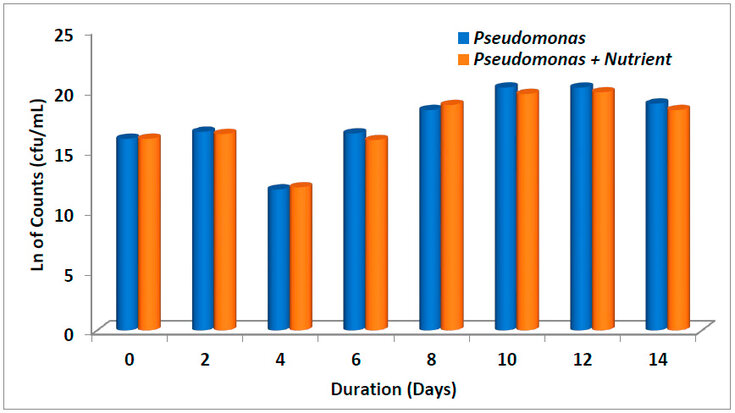
Figure. 5: Average bacterial counts in different treatments over 14 days
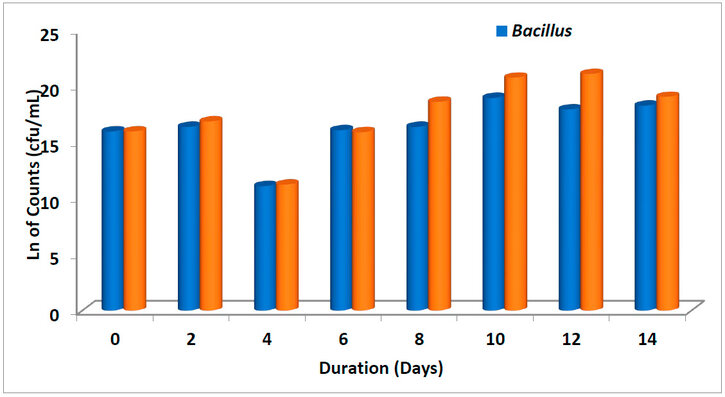
Figure. 6: Average bacterial counts in different treatments over 14 days
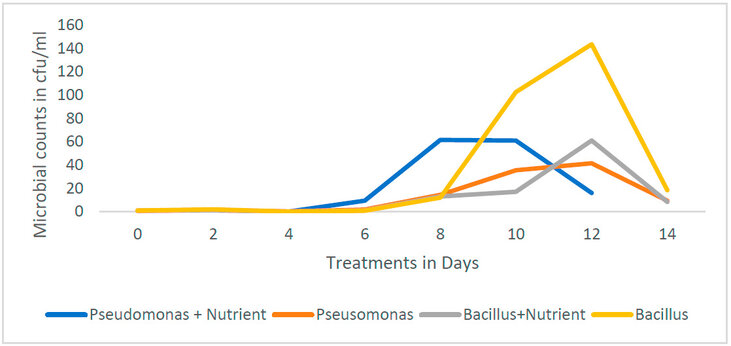
Figure 7: Bacterial growth curve for the different treatments
DISCUSSION
The influence of environmental factors rather than the genetic capability of a microorganism has been reported to limit the degradation of pollutants.34 pH is an essential factor influencing microbiological metabolic activity and microorganism growth. Different microorganisms can grow over a wide pH range, and organisms have their tolerance levels. Studies have shown that fat, oil, and grease-degrading organisms have optimum growth between pH 5.5 and 8.0, with maximization at 7.5. 35 From the observed results in this study (Table 1), the isolates actively degraded the hydrocarbons at approximately pH 6. According to Bala et al. and Xu et al., the influence of environmental factors rather than the genetic capability of a microorganism has been reported to limit the degradation of pollutants 34,19. According to Chen et al., the co-existence of hydrocarbon-degrading microbial consortia may increase the degradative efficiency 36, with greater tolerance to acidic pH and subsequent decontamination of polluted soils.
The results from Table 3 show the comparison of the initial concentrations of LPFO and their final concentrations after 14 days of treatment. It can be observed that the susceptibility of the hydrocarbon compounds to microbial degradation varies with the type and size of the hydrocarbon molecule. In line with the findings of Lina et al. and Liu et al., alkanes of intermediate chain length (C10–C24) were degraded rapidly compared to very long chain alkanes (C20–C34) 37, 38. According to Nzila35 and Constanza39, aliphatic compounds are degraded as linear aliphatic compounds > branched aliphatic compounds >cyclic aliphatic compounds, respectively. Nzila et al. observed that the biodegradation of short and middle-chain aliphatic compounds is more extensive than the long-chain hydrocarbons 40.
There was a significant increase in degradation when the LPFO was inoculated with B. licheniformis and Pseudomonas aeruginosa. Ferreira et al. and Nayak et al. reported the ability of B. licheniformis and other Bacillus sp to degrade engine oil. 41,42
Adding inorganic or organic nitrogen-rich nutrients (biostimulation) is a practical approach to enhance the bioremediation process.42 Positive effects of nutrient amendment on microbial activity and/or petroleum hydrocarbon degradation have been widely demonstrated.43,44 However, in this study, there was no significant effect of nutrient amendment on hydrocarbon degradation compared to when the individual microorganisms acted alone.
The degradation of n-alkanes higher than C9 increases with the chain length.45 Various microorganisms readily degrade the longer chain aliphatic hydrocarbons under aerobic conditions. However, in this study, the n-alkanes C19 showed resistance to degradation by both the organisms and when amended with nutrients. According to Pandolfo and Ghosal et al., the ability to degrade polycyclic aromatic hydrocarbons or their fractions is not limited to particular species but occurs over a broad group of bacterial strains 46, 47 and increasing the microbial consortia involved in this study may increase the degradation 36 of the n-alkanes C19. Xu et al. noted that individual organisms often prefer to metabolize a limited range of hydrocarbon substrates. That may be the reason for the observed non-degradation of C19. 48
There was an increase in the bacterial count of B. licheniformis, followed by Pseudomonas aeruginosa during the degradation process, which demonstrated the ability to utilize LPFO as this organism's energy source. Wang et al. reported an increase in the cell number of B. stearothemophilus during the degradation process of engine oil.49 Microorganisms have different rates at which they utilize and degrade hydrocarbons in the soil or water.50 This rate is reflected in the multiplication of isolated organisms and colony-forming units (cfu). According to Bala et al., the number of microbes that use the contaminants as carbon and energy sources increases during active contaminant biodegradation. 34
The average bacterial counts of samples with different treatment options are shown in Figures 5 and 6, respectively. The lowest bacterial counts were observed on the 4th day in all the treatments (Bacillus, Bacillus + nutrient and Pseudomonas, Pseudomonas + nutrient, respectively). This was also observed as we studied the microbial growth curve of different treatments (Figure 7). Highest bacterial count was observed when treated with Bacillus + nutrient on the 10th and 12th day of treatment. As observed in this study, the decline in the population of degrading microbes might be due to inhibitory metabolites 51, which are produced during the degradation of LPFO. Heras-Martínez et al. reported that degradation increased with the incubation period and peaked after 30 days, where nearly 91% of the hydrocarbon was degraded. 51
CONCLUSIONS
Based on the observations made in this study, it was concluded that Bacillus licheniformis could degrade LPFO than Pseudomonas aeruginosa. It was also observed that degradation increased with the incubation period, and on average, 99.9% of the hydrocarbon was degraded. It can be observed that the susceptibility of the hydrocarbon compounds to microbial degradation varies with the type and size of the hydrocarbon molecule. Interestingly, adding nutrient supplements showed no remarkable increase in LPFO degradation. Further work may include a field study using a diverse microbial consortium to ascertain the synergistic effect of different microorganisms. Such studies may also include bioassays on the potential toxicity of degradation products.
Author Contributions: Conceptualization, Chinedu Emeka Ihejirika, Joseph Ifeanyichukwu Garricks and Ejeagba Okorie Imo; methodology, Chinedu Emeka Ihejirika, and Ejeagba Okorie Imo; software, Joseph Ikechukwu Nwachukwu; validation, Chinedu Emeka Ihejirika, and Ejeagba Okorie Imo; formal analysis, Ihuoma Ezichi Mbuka-Nwosu; investigation, Chinedu Emeka Ihejirika; resources, Etienne Chukwuma Chinakwe; data curation, Obenade Moses.; writing—original draft preparation, Chinedu Emeka Ihejirika and Ejeagba Okorie Imo.; writing—review and editing, Chinedu Emeka Ihejirika and Ejeagba Okorie Imo; visualization, Christopher Chibuzor Ejiogu; supervision, Ursula Ngozi Nwaogwugwu; project administration, Chinedu Emeka Ihejirika, Joseph Ifeanyichukwu Garricks and Ejeagba Okorie Imo.; funding acquisition, Joseph Ifeanyichukwu Garricks. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Acknowledgments: We express our profound gratitude to the Laboratory Technologists of the Department of Environmental Management, Federal University of Technology, Owerri, Imo State, Nigeria, for providing technical support during the study.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Koshlaf E., Ball AS (2017) Soil bioremediation approaches for petroleum hydrocarbon polluted environments. AIMS Microbiology.2017, 3, pp.25-49.
2. Mohanty S., Jasmine J., Mukherji S. (2013) Practical considerations and challenges involved in sur factant enhanced bioremediation of oil. Bio Med Res. Interl.2013, 2314-6133.
3. Maletic S., Dalmacija B., Roncevic S. Petroleum Hydrocarbon Biodegradability in Soil Implications for Bioremediation. InTech. 2013.
4. Wolejko E., Wydro U., Loboda T. "The ways to increase the efficiency of soil bioremediation,"
5. Ecol of Chem Engi Study,2016, 23,155-174.
6. Kebede G., Tafese T., Abda E.M., Kamaraj M., Assefa F. Factors Influencing the Bacterial Bio remediation of Hydrocarbon Contaminants in the Soil: Mechanisms and Impacts. Jour of Chem. 2021, 2090-9063.
7. Waziri M., Audu A., Alex B. (2014) A Comparison of the Quality of Low Pour Fuel Oils (LPFO) Blended From Nigerian and Foreign Crude Oils, Petro Sci and Technol,2014, 32, 1473-1479,
8. Alhassan H.M., Fagge S.A. (2013) Effects of Crude Oil, Low Point Pour Fuel Oil and Vacuum Gas Oil Contamination on the Geotechnical Properties Sand, Clay and Laterite Soils. International Journal of Engineering Research and Applications, 2013, 3, 1947-1954.
9. Ohijeagbon I.O., Jekayinfa S.O., Waheed M.A. (2012) "Assessing the emission factors of low-pour
fuel-oil and diesel in steam boilers", International Journal of Development and Sustainability, 2012, 1, 688-700
10. Otunyo A.W., Anele O. Effect of Waste Engine Oil Contamination on Geotechnical Propertie of Clay Soil. European International Journal of Science and Technology. 2015, 4, 28-38.
11. Joanne, M. W., Kathleen, M.S. and Dorothy, H. W. Prescourt's Microbiology. (11th edition). London: McGraw Hill, 2017
12. Ehis-Eriakha C.B., Akemu S.E., Otumala S.O., Ajuzieogu C.A. Biotechnological Potentials of Microbe Assisted Eco-Recovery of Crude Oil Impacted Environment. IntechOpen Book Series.Haider, 2021.
13. FU, Ejaz M., Cheema S.A., Khan M.I., Liqun B.Z.C., Salim M.A., Naveed M., Khan N., Núñez-Delgado A., Mustafa A. Phytotoxicity of petroleum hydrocarbons: Sources, impacts and remediation strategies, Environmental Research, 2021, 197,111031,
14. Adeniran M.A., Oladunjoye M.A., Doro K.O. (2023) Soil and groundwater contamination by crude oil spillage: A review and implications for remediation projects in Nigeria. Frontiers of Environmental Science, 2023, 11, 1137496.
15. Allison E., Mandler B. Petroleum and the Environment, Part 14/24. Spills in Oil and Natural Gas Fields. American Geosciences Institute, 2018.
16. Bekele G.K., Gebrie S.A., Abda E.M. (2023) Kerosene Biodegradation by Highly Efficient Indigenous Bacteria Isolated From Hydrocarbon-Contaminated Sites. Microbiology Insights, 2023, 16.
17. Sharma I. Bioremediation Techniques for Polluted Environment: Concept, Advantages, Limitations, and Prospects. In: Trace Metals in the Environment - New Approaches and Recent Advances. Intechopen, 2020.
18. Mojarad M., Alemzadeh A., Ghoreishi G., Javaheri M. (2016). Kerosene biodegradation ability and characterization of bacteria isolated from oil-polluted soil and water, Jou. of Env Chem Eng, 4(4) Part A: 2016, 4323-4329,
19. Alori E.T., Gabasawa A.I., Elenwo C.E., Agbeyegbe O.O. (2022) Bioremediation techniques as affected by limiting factors in soil environment. Frontiers of Environmental Science. 2022, 2, 937186.
20. Xu A., Zhang X., Wu S., Xu N., Huang Y., Yan X., Zhou J., Cui Z., Dong W. (2021) Pollutant Degrading Enzyme: Catalytic Mechanisms and Their Expanded Applications. Molecules. 2021, 26, 4751.
21. Xue L., Chongling F., Min L., Kun L., Lingyu W., Renguo L., Yuanyuan L., Yining H. (2022) "Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review" Open Chem, 2022, 20, 793-807.
22. Abatenh E., Gizaw B., Tsegaye Z., Wassie M. (2017). The Role of Microorganisms in Bioremediation- A Review. Open J of Env Bio, 2017, 2, 038–046.
23. Koh L.M., Khor SM (2022) Biodegradation Process: Basics, Factors Affecting, and Industrial Applications. In: Ali, G.A.M., Makhlouf, A.S.H. (eds) Handbook of Biodegradable Materials. Springer Cham, 2022
24. Aikaterini Z., Eleni K., Fotini S., Evangelos G. Monitoring the biodegradation of TPH and PAHs in refinery solid waste by biostimulation and bioaugmentation, Jou of Env Chem Eng, 2022, 7, 103054.
25. Bushnell L.D., Haas H.F. The Utilization of Certain Hydrocarbons by Microorganisms. Journal of Bacteriology. 1941, 41, 653-73.
26. Wang J.J., Shih J.C.H. (1999) Fermentation production of keratinase from Bacillus licheniformis PWD-1 and a recombinant B.subtilis FDB-29. Journal of Industrial Microbiology and Biotechnology, 1999, 22, 608-616.
27. Al-Dhabaan F.A. (2019) Morphological, biochemical and molecular identification of petroleum hydrocarbons biodegradation bacteria isolated from oil polluted soil in Dhahran, Saudi Arabia. Saudi Journal Biological Sciences; 2019, 26,1247-1252.
28. S.O. Sojinu and O. Ejeromedoghene. Environmental challenges associated with processing of heavy crude oils. Intechopen. 2019, 1-15.
29. Cruickshank R., Dugid J.P., Marmoin B.P., Swain R.H.A. Medical Microbiology. Vol. 2. The Practice of Medical Microbiology. 13th ed.Churchill Livingstone, Edinburgh. 1982, pp. 273-284.
30. Koneman's Color Atlas and Textbook of Diagnostic Microbiology. Procop, Gary W. Published by Jones & Bartlett Learning, 2016.
31. Cheesbrough M. Medical Laboratory Manual for Tropical Countries: Microbiology. ELBS Edition, Cambridge, 1991, 196-205.
32. Edison T.E., Cruz D., Martin J., Torres O. Gelatin hydrolysis test protocol. American Society for Microbiology, 2012.
33. APHA (2017). Standard Methods for the Examination of Water and Wastewater (23rd ed.). Washington DC: American Public Health Association.
34. Allred D., Edwards H., Klemme Rogers, Wulf., Hodge. (1963) Proposed procedures for microbiological examination of fuels. SIM Special Publications, No. 1. Merck, Sharp & Dohme Research Laboratories, Rahway, NJ 1963.
35. Bala S., Garg D., Thirumalesh B.V., Sharma M., Sridhar K., Inbaraj B.S., Tripathi M. Recent Strategies for Bioremediation of Emerging Pollutants: A Review for a Green and Sustainable Environment. Toxics. 2022, 10, 484.
36. Nzila A., Razzak SA, Sankara S., Nazal M.K., Al-Momani M., Kang G.U., Ibal J.C., Shin J.H. Characterisation and microbial community analysis of lipid utilizing microorganisms for biogas formation. PLoS One. 2019, 14.
37. Chen R., Zhao Z., Xu T., Jia X. Microbial Consortium HJ-SH with Very High Degradation Efficiency of Phenanthrene. Microorganisms. 2023, 23;11(10):2383.
38. Lina L., Jie L., Yu C., Zhimao M., Lin W., Qiqi L., Si Z. Degradation potential of alkanes by diverse oil-degrading bacteria from deep-sea sediments of Haima cold seep. Frontiers in Microbiology. 3.
39. Liu H., Xu J., Liang R., Liu J. Characterization of the Medium- and Long-Chain n-Alkanes Degrading Pseudomonas aeruginosa Strain SJTD-1 and Its Alkane Hydroxylase Genes. PLoS ONE. 2014,9(8)
40. Constanza C.M., Roberto E.D., Lisette H., Laura R., Bárbara B., Flavia D., Michael S. Bioremediation of Petroleum, Reference Module in Life Sciences, Elsevier. 2019.
41. Nzila A. Current Status of the Degradation of Aliphatic and Aromatic Petroleum Hydrocarbons by Thermophilic Microbes and Future Perspectives. International Journal of Environmental Research and Public Health. 2018, 15, 2782.
42. Ferreira R.M., Ribeiro B.D., Stapelfeldt D.M.A., do Nascimento R.P., Moreira M.de.F.R. Oil biodegradation studies with an immobilized bacterial consortium in plant biomass for the construction of bench-scale bioreactor, Cleaner Chem Eng 2023, 6,100107.
43. Nayak N.S., Purohit M.S., Tipre D.R., Dave S.R. Biosurfactant production and engine oil degradation by marine halotolerant Bacillus licheniformis LRK1, Biocatalysis and Agri Biotech., 2020, 29:101808.
44. Udume O.A., Abu G.O., Stanley H.O., Vincent-Akpu I.F., Momoh Y., Eze MO Biostimulation of Petroleum-Contaminated Soil Using Organic and Inorganic Amendments. Plants. 2023, 12, 431.
45. Kanwal M., Ullah H., Gulzar A., Sadiq T., Ullah M. Biodegradation of Petroleum Hydrocarbons and The Factors Effecting Rate of Biodegradation. American Journal of Biomedical Sci & Res.2022, 16.
46. Medic A., Ljesevic M., Inui H., Beskoski V., Kojic I., Stojanovic K., Karadzi' I. Efficient biodegradation of petroleum n-alkanes and polycyclic aromatic hydrocarbons by polyextremophilic Pseudomonas aeruginosa san ai with multidegradative capacity. Royal Society of Chemistry Advances. 2020.
47. Pandolfo E., Barra C.A., Rolando L. Recent Advances in Bacterial Degradation of Hydrocarbons. Water. 2023, 15, 375.
48. Ghosal D., Ghosh S., Dutta T.K., Ahn Y. (2016) Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Frontiers in Microbiology. 2016, 7:1369.
49. Xu X., Liu W., Tian S., Wang W., Qi Q., Jiang P., Gao X., Li F., Li H., Yu H. (2018) Petroleum Hydrocarbon-Degrading Bacteria for the Remediation of Oil Pollution Under Aerobic Conditions: A Perspective Analysis. Frontiers in Microbiology. 2018, 9:2885.
50. Wang D., Lin J., Lin J., Wang W., Li S. (2019) Biodegradation of Petroleum Hydrocarbons by Bacillus subtilis BL-27, a Strain with Weak Hydrophobicity. Molecules. 2019 24, 3021.
51. Zuo L. Bioremediation of Crude-Oil Polluted Soil Using Immobilized Microbes. IOP Conf. Ser.: Earth Envil Sci. 2020, 510, 042047
52. Heras-Martínez H.M., Muñoz-Castellanos L.N., Bugarin A., Ramos‑Sánchez V.H., Ochoa I.S. Chávez-Flores D. Biodegradation of Polycyclic Aromatic Hydrocarbons by Acremonium Sp. Activity. Revista Internacional de Contaminación Ambiental. 2022
53. Chen R., Ren L., Shao J., He Y., Zhang X. Changes in degrading ability, populations and metabolism of microbes in activated sludge in the treatment of phenol wastewater. RSC Adv. 2017, 7, 52841-52851
Received: June 5, 2024 / Accepted: September 3, 2024 / Published: December 15, 2024
Citation: Ihejirika C E, Ifeanyichukwu Garricks J, Okorie Imo E, Ikechukwu Nwachukwu J, Ezichi Mbuka-Nwosu I, Chukwuma Chinakwe E, Ngozi Nwaogwugwu U, Chibuzor Ejiogu C, Moses O. Biodegradation efficiencies of Low Pour Fuel Oil by Pseudomonas aeruginosa and Bacillus licheniformis isolates. Bionatura journal. 2024;1(4):15. doi: 10.70099/BJ/2024.01.04.15
Additional information Correspondence should be addressed to basailmilagros@gmail.com
Peer review information. Bionatura thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
ISSN.3020-7886
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Publisher's Note: Bionatura Journal stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2024 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




