Promising organic compounds in invasive aquatic plants identified in freshwater lagoons in Cuba
Leslie Hernández-Fernández 1*, Claudia Linares Rivero 2, Janet Quiñones Galvez 3,
José Carlos Lorenzo Feijoo4, Yanier Acosta Fernández5, Roberto González-De Zayas6
1 Centro de Bioplantas (Universidad de Ciego de Ávila, Máximo Gómez Báez/ Ciego de Ávila/ Cuba); coraleslhf@gmail.com.
2 Centro de Bioplantas (Universidad de Ciego de Ávila, Máximo Gómez Báez/ Ciego de Ávila/ Cuba); claudia911007@gmail.com.
3 Centro de Bioplantas (Universidad de Ciego de Ávila, Máximo Gómez Báez/ Ciego de Ávila/ Cuba); janetquinonesgalvez@gmail.com.
4Centro de Bioplantas (Universidad de Ciego de Ávila, Máximo Gómez Báez/ Ciego de Ávila/ Cuba); lorenzojosecarlos68@gmail.com.
5 Centro de Bioplantas (Universidad de Ciego de Ávila, Máximo Gómez Báez/ Ciego de Ávila/ Cuba); yacfdez@gmail.com.
6 Facultad de Ciencias Técnicas (Universidad de Ciego de Ávila, Máximo Gómez Báez/ Ciego de Ávila/ Cuba); roberto.gz710803@gmail.com.
* Correspondence: coraleslhf@gmail.com;
ABSTRACT
Pistia stratiotes L. and Eichhornia crassipes (Mart.) Solms have promising organic compounds. These species are invasive aquatic plants present in some freshwater lagoons in Cuba. Using its phytochemical properties can be a way out of its negative impact. Water quality from two lagoons was evaluated. The content of phenols, anthraquinones and flavonoids were evaluated for both species. The extraction was made with ethanol (90 %), and the High-Performance Thin-Layer Chromatography analytical technique was used for the phytochemical test. Both lagoons were highly polluted. E. crassipes were observed in two forms: dense and not dense stands. The content of phenolic compounds ranged from 3.07 to 9.2 mg g-1 DW, the anthraquinones ranged from 1.06 to 3.74 mg g-1 DW and flavonoids ranged from 33.9 to 18.2 mg g-1 DW. The highest content of organic compounds was recorded in the dense stands of E. crassipes. Some coincidences for morindin and rubiadin standards were found in P. stratiotes. Coincidences for kaempferol and quercetin standards were also found in the samples. The results of this study suggest that both species of plants could be used as a source of organic compounds by the pharmaceutical industry of Cuba.
Keywords: freshwater lagoon; invasive plants; phenols; anthraquinones; flavonoids
INTRODUCTION
In Cuba, programs are being developed to prevent and control invasive species at the national and local levels since their harmful effects on native species, ecosystems, and ecosystem services are widely recognized1. Specifically, the invasive plants have great potential to disrupt global biodiversity, forestry, livelihood, and human health2. Nevertheless, ecological and socio-economic investigations of invasive plants to facilitate restoration strategies are insufficient in many species 2.
Pistia stratiotes L. and Eichhornia crassipes (Mart.) Solms are invasive, noxious, and transformative freshwater aquatic plants1. Despite the negative impacts on aquatic ecosystems, these invasive plants can be beneficial. Some authors have found secondary metabolites such as phenols, anthraquinones, and flavonoids, among others, with antibacterial3,4, antifungal5,6,7, antiviral8, anticancer9,10, antioxidant11,12,13,14 and bioacaricide15 properties.
In Cuba, studies on aquatic flora are limited16. There are few studies on P. stratiotes and E. crassipes17,18,19. No references have been found on the content of secondary metabolites present in these species. Given the negative impact caused by P. stratiotes and E. crassipes in the lagoons where they predominate and the need to find solutions to it, this study aimed to identify promising organic compounds present in leaves and roots of P. stratiotes and E. crassipes. These results could benefit the pharmaceutical industry in Cuba.
MATERIAL AND METHODS
Study area
The province of Ciego de Avila is located in Central Cuba. P. stratiotes is present at Vista Alegre lagoon (L.1) (0.013 km2). It is located in the western part of the city of Ciego de Avila (21°51’9’’N-78°46’39’’W) and E. crassipes is present at La Turbina lagoon (L.2) (0.086 km2), located in the outskirts of the city (21°50’51’’N-78°45’43’’W).
Analysis of physicochemical and microbiological of the water
Some physicochemical and microbiological parameters were analyzed to evaluate the water quality of both lagoons. For water analyses, samples were taken at one site in L.1 and three in L.2. The number of samples corresponded with the area occupied by each la-goon. Water samples were taken in the morning (from 08:00 to 09:00 am). To evaluate physicochemical parameters, the water samples were collected in polyethylene bottles, previously washed with hydrochloric acid, and sterilized glass bottles to evaluate microbiological parameters. Some of these parameters (pH, electric conductivity, oxygen saturation, chemical demand of oxygen, and fecal coliforms) were used to calculate the Water Quality Index (WQI)20

Where:
Wi: relative weight of each indicator or parameter.
qi: value (in percentage) obtained from correlation functions.
Analysis of organic compounds
According to Coetzee et al.21, vast amounts of E. crassipes grow near the margins of the lagoons with elongated petioles (up to 1 m tall) (dense stand); however, in sparse infected sites and at the edge of such sites, the petioles are bulbous and short (less than 30 cm) (not dense stand).
The samples of leaves and roots were washed with fresh water, and subsequently with distilled water before drying (constant weight) in a certified oven at 70 °C for 48 hours. The dry samples were powdered to approximately 2 µm in size. The method of solid-liquid maceration was used to obtain the extracts of the studied plants. For the preparation of ethanolic extract, 5 g of the powdered dry mass of plant was placed in 150 mL of ethanol (90 %) (v:v), used as an organic solvent, with a solid-liquid ratio of 1:30 (m:v).
Total Phenolic Content (TPC) was determined using the colorimetric method Folin–Ciocalteu, proposed by Gurr, McPherson and Bowles (1992) in Rivero22, in Total Anthraquinone Content (TAC) was determined according to the colorimetric method described by Han et al.23. For the calculated result, the molar extinction coefficient of 5500 M-1cm-1 about alizarin and proposed by Schulte24 was used. Total Flavonoid Content (TFC) was determined using the colorimetric method proposed by Kim et al.25. The result was expressed as mg per g of dry mass (mg g-1 DW).
High Performance Thin-Layer Chromatography (HPTLC)
The identification of phenolic acids, anthraquinones, and flavonoids using High-Performance Thin-Layer Chromatography (HPTLC) was made for both plants (leaves and roots) and in the case of E. crassipes in both stands (dense and not dense). HPTLC Plates ALUGRAM® Nano Silica Gel, 8.0 x 10.0 cm, was used to identify the said substances. The plates were activated with oxalic acid (1 %) (m:v) at 100 °C for 2 minutes. Applying samples (10 µL of extract) was performed over each lane at 0.5 cm between each sample and 1.0 cm at the edges. The solvents used for these analyses were toluene, ethyl acetate, methanol, and formic acid in the ratio 32: 14: 12: 5.
Some anthraquinones were used as reference standards: lucidine, alizarin, rubiadin, 1 methyl ether, damnacanthal, emodin, rubiadin, chrysophanic acid and morindine. Some flavonoids were also used, as well as phenolic acids such as quercetin, kaempferol, rutin and gallic acid and the iridoids asperolosidic acid, diacetyl asperolosidic acid. The visualization was carried out at 254 and 366 nm and the retention factor (Rf) was calculated as the quotient between the migration distance by compound from the application point and the migration distance of the solvent front.
Statistical analysis
The TAC, TPC, and TFC data in all the samples were subject to variance analysis using the Kruskal – Wallis non-parametric test. When significant differences from the Kruskal -Wallis test were found, the Wilcoxon test was used to determine the samples with such differences. Statistical analyses were made using the R software version 3.1.2 (R Core Team 2014), package Vegan26.
RESULTS
Analysis of physicochemical and microbiological of the water
The pH of water was lower at L.1 than at L.2, so dissolved oxygen and total hardness. Other physicochemical parameters were higher at L.2 (Table 1).
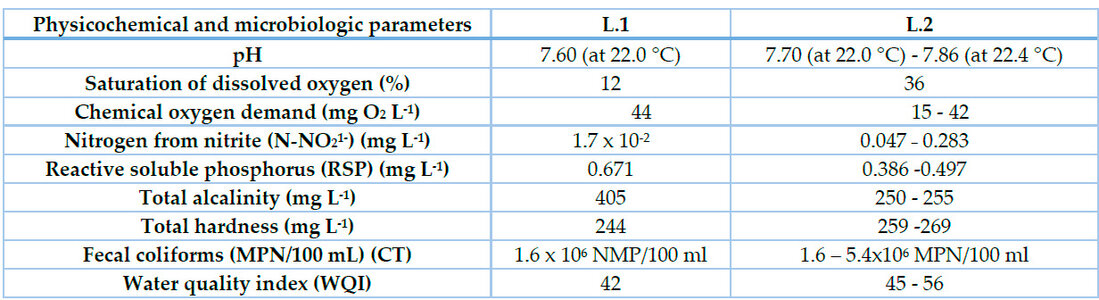
Table 1. Physicochemical and microbiological conditions of freshwater lagoons L.1 and L.2
Analysis of organic compounds
The TPC ranged from 3.07 to 9.2 mg g-1DW. The highest mean TPC was found in the roots of the dense stand of E. crassipes (9.19 mg g-1DW) without significant differences with the leaves of the dense stand and the leaves of the not dense stand of E. crassipes (Fig. 1a). The TAC ranged from 1.06 to 3.74 mg g-1 DW. The highest mean of TAC was recorded for the leaves of E. crassipes (dense stand) (3.73 mg g-1DW), followed by the leaves of the not dense stand of E. crassipes sample. The lowest TAC was found in the leaves of P. stratiotes (Fig. 1b). The TFC ranged from 18.2 mg g-1 to 33.9 mg g-1DW. The highest mean of TFC was found in the leaves of the dense stand of E. crassipes (33.92 mg g-1DW), without significant differences with the leaves of the not dense stand (Fig. 1c).

Figure 1. Total Phenolic Content (A) (Kruskal-Wallis chi-squared = 102.36, df = 5, p-value < 2.2e-16). Total Anthraquinone Content (B) (Kruskal-Wallis chi-squared = 47.046, df = 5, p-value = 5.559e-09). Total Flavonoid Content (C) (Kruskal-Wallis chi-squared = 34.244, df = 5, p-value = 2.129e-06) (mg g-1 DW) in ethanolic extracts of the leaves (HP) and roots (RP) of P. stratiotes, leaves (HED) (HEND) and roots (RED) (REND) of the dense and not dense stand of E. crassipes. Letters in the above quartile indicate significant differences among extracts.
High Performance Thin-Layer Chromatography (HPTLC)
Using unrevealed UV365nm (Fig. 2a), the phytochemical composition of extracts from leaves (HP) and roots (RP) of P. stratiotes showed similarities in two bands of both parts of the plant (light blue) and (blue-violet) (Fig. 2a). The blue bands were only visible in the leaves of P. stratiotes. In ethanolic extracts of E. crassipes (Fig. 2a), the behavior was similar in the leaves of the dense and not-dense stands, with different shades of pink. However, a blue band was observed in HEND that was not present in HED, which could be related to the plant's development stage. For the root samples of E. crassipes, results were similar in both stands, with four bands of different shades of blue and a green band.

Figure 2. Phytochemical screening using HPTLC in ethanolic extract of leaves (HP) and roots (RP) of P. stratiotes, leaves (HED) and roots (RED) of dense stands of E. crassipes and leaves (HEND) and roots (REND) of not dense stands of E. crassipes. Chromatographic plates run in the solvent IV system. (A) Unrevealed Ultraviolet Light (UV365 nm), (B) Revealed white light with KOH, (C) Revealed ultraviolet light (UV365 nm) with KOH, (D) Revealed ultraviolet light (UV365 nm) with NP and (E) Revealed white light with V/S). Mixtures of standards morindine, diacetyl asperolosidic acid, rutin and Gallic acid (MZ1). Mixtures of standards lucidin, rubiadin-1 methyl ether, emodin, alizarin, damnacanthal and chrysophanic acid (MZ2). Mixtures of standards gallic acid, quercetin and kaempferol (MZ3).
In the chromatogram revealed with KOH and in white light (Fig. 2b), a brown band was observed in the ethanolic extracts, which coincides with standards for quercetin and kaempferol compounds. In HP and RP extracts, this brown band was also present but barely noticeable, which could indicate less content of these compounds. A yellow band coincident with moridina standards was observed in the HP extract.
The chromatogram revealed with KOH (Fig. 2c) at UV365nm showed the bands observed when white light was used. Some other bands (linked to E. crassipes) were observed: four green–blue bands in the roots of plants from both stands (dense and not dense), and of these four bands, only one was observed in the leaves (at HED and HEND).
The screening using UV365nm revealed NP (Fig. 2d) showed a barely noticeable yellow-orange band that coincided with rutin and morindine standards. A similar band was present in the ethanolic extract RP but was scarcely perceptible.
Three green-blue bands were observed in the ethanolic extracts of HED and HEND, one more intense in HEND and the other two bands of the same color. These green-blue bands were not present at the roots of E. crassipes. In the roots of E. crassipes (in both stands), a blue band that was not in the leaves of this plant was present.
The plates revealed in white light and with V/S showed a brown band (Fig. 2e) in all studied extracts that coincided with quercetin and kaempferol standards. It was unclear whether the color coincided when both standards were run at the same Rf, showing a mixture between yellow and brown due to the overlap between both compounds, which did not allow bands to be defined. One olive green band was observed in all extracts (more intense in HED and RED) except for the RP sample. This band was observed at the same Rf as the diacetyl asperolosidic acid standard.
The phytochemical screening of ethanolic extracts of both studied plants, the use of solvent system IV, and three revealers (KOH, NP, and V/S) showed coincidences in Rf and color with morindine or rubiadin in the leaves and roots of P. stratiotes. Some coincidences in Rf and color with quercetin, kaempferol, and diacetyl asperolosidic acid standards were found, except for the roots of P. stratiotes.
DISCUSSION
This is the first study on the water quality of freshwater lagoons where both invasive plants live. The pH of these lagoons was near the pH of previous reports for Cuban freshwater lagoons20. In both lagoons, its oxygen saturation was low, with a clear tendency to hypoxic conditions. This low-dissolved oxygen content was highly related to the high content of organic matter expressed as chemical oxygen demand (COD) that was above the limits of the Cuban standard for water quality (NC.25 in Pelegrín-Morales et al.)27. Considering these limits (COD), the water quality of these lagoons could be classified as “poor”. The causes of the high content of organic matter at both lagoons could be related to sewage discharges from neighboring dwellings and to the death of plants that end at the bottom of the lagoons. P. stratiotes and E. crassipes completely cover the water surface of L.1 lagoon covers almost 30 % of L.2. The decomposition of dead plants at the bottom of these lagoons triggers a significant oxygen uptake by bacteria, which results in the low content of dissolved oxygen in the lagoon waters28. Another evidence of sewage disposal into these lagoons is the high contents of phosphorus and nitrite, mainly in L.2. Due to the alkalinity of both lagoons, their waters could be classified as waters with temporal hardness and not sulfated, like the groundwaters, rivers, and dams of this region and Cuba29.
In Latin America, studies of organic compounds in E. crassipes and P. stratiotes are scarce30, and in the case of Cuba, no reference to this topic has been found. Some studies around the world reported phenolic compounds in both plants, which vary when comparing them with the results of this study9,6. López et al.4 obtained lower TPC in the leaves (2.36 mg g-1 DW) and the roots (2.7 mg g-1 DW) of E. crassipes than the TPC found in this study.
Like TPC, TAC was also determined in both plants. However, Tulika and Mala8 detected TAC only in E. crassipes, not P. stratiotes. Tyagi and Agarwal29 did not detect TAC in the roots of E. crassipes. Tyagi32 found no TAC in E. crassipes leaves with methane extracts. This author did not find TAC with ethanolic extracts. The same author32 studied TAC in P. stratiotes and detected it in the leaves and roots with methanolic and ethanolic extracts. López et al.4 detected (qualitatively) TAC in the leaves and roots of ethanolic extracts of E. crassipes.
In our samples, flavonoids were present. However, Rodríguez et al.5 did not find these compounds in the leaves of E. crassipes and Vargas and Zambrano3 neither in E. crassipes. López et al.4 recorded lower TFC than the TFC recorded in this study in the leaves (0.75 mg g-1 DW) and roots (0.29 mg g-1 DW) of E. crassipes. The TFC in P. stratiotes and E. crassipes was higher than TAC and TPC, which coincides with the results obtained by Sudirman et al.33.
Although this study did not focus on the relationship between the environmental conditions of freshwater lagoons and the content of organic compounds in plants, some authors have suggested the influence of these conditions and other factors such as solvent extraction, season of the year, the age of the plant32,34,4,10, in the content of organic compounds in P. stratiotes and E. crassipes. This study demonstrated that the growth state of E. crassipes (dense and non-dense) also influences the content of organic compounds. For E. crassipes, TAC was higher in the dense stand than in the not dense stand (leaves and roots) and similar values of TAC and TFC were found in both stands of the same lagoon (L.2). Another phase of this research could be an evaluation of how the environmental conditions of each studied lagoon affect the content of organic compounds in both plants.
Identifying some organic compounds using the HPTLC technique showed results that differed from other studies of the same species. For instance, our results showed the presence of quercetin and kaempferol in diacetyl asperolosidic acid. However, Shanab et al.35 did not find these compounds in the Nile's methanolic extracts of E. crassipes. Using the same extraction solvent (ethanol), Souza36 identified only anthocyanins in the leaves of E. crassipes. López et al.4 identified gallic acid (a phenolic compound) and catechin (flavonoid) in E. crassipes. Khalid et al.6 and Gebrehiwot et al.10 found terpenoids and flavonoids, including quercetin and kaempferol and evaluated their antifungal and antibacterial properties. Gebrehiwot et al.10, stated that as antimicrobial activity, the concentrations of E. crassipes extracts can vary from 20 μg mL-1 to 500.0 μg mL-1, depending on the plant organ used. Rufchaei et al.37 determined gallic acid in E. crassipes (258.3 ± 10.8 mg g-1) and evaluated their antibacterial activities against Escherichia coli and Pseudomonas aeruginosa.
Other non-aquatic invasive plants identified in Cuba have potential for use in agriculture, the food industry and pharmaceuticals, such as Tithonia diversifolia (Hemsl.) A. Gray1,2. This species was studied in Cuba by Herrera et al.38 who demonstrated the positive effect of climatic factors on the concentration of secondary metabolites, such as phenols. Also, the species Senna spectabilis (Lam.) Irwin & Barneby is an invasive plants, identified in Cuba1, in which Prajitha and Bai39 identified fifteen important allelochemicals, including phenolic compounds, flavonoids, anthraquinone. However, no studies carried out in Cuba on this subject were found. Therefore, an explicit assessment of P. stratiotes and E. crassipes, in Ciego de Ávila province, can enable a cost-benefit analysis to help determine its ecological economics while pursuing the targets of the sustainable development and your ecosystems restoration2. In this regard, these results can be extended to the rest of the country. To do this, the biological activity of the compounds identified in P. stratiotes and E. crassipes must be evaluated, and the extraction and purification methods must be optimized.
CONCLUSIONS
Today, the high cost of cleaning these freshwater lagoons and the potential pollution of groundwater is the main reason why the management and use of P. stratiotes and E. crassipes must be an important issue to be solved by government authorities. The results of this study show that in P. stratiotes and E. crassipes, the content of phenolic compounds varied from 3.07 to 9.2 mg g-1, anthraquinones from 1.06 to 3.74 mg g-1 and flavonoids from 33.9 to 18.2 mg g-1 of dry weight, so the use of their extracts in the pharmaceutical industry may be an alternative to manage and control their invasive effects. However, research is required on their antioxidant effects and their antifungal and antibacterial properties.
Author Contributions: Leslie Hernández-Fernández: conceptualization, visualization, formal analysis, investigation, writing-original draft preparation, project administration. Claudia Linares Rivero: formal analysis, investigation, writing-review and editing. Janet Quiñones Gálvez: formal analysis, investigation, writing-review and editing. José Carlos Lorenzo Feijoo: formal analysis, writing-review. Yanier Acosta Fernández: formal analysis, writing-review. Roberto González de Zayas: conceptualization, visualization, formal analysis, writing-review. All authors have read and agreed to the published version of the manuscript." Please turn to the CRediT taxonomy for the term explanation.
Funding: This research received no external funding.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Data Availability Statement: The study did not report any data.
Acknowledgments: These results respond to Territorial Project (PT: 121CA003-005) “Evaluation of the use and management of invasive aquatic plants Pistia stratiotes L. and Eichhornia crassipes (Mart.) Solms as an alternative for their use in urban agriculture in Ciego de Ávila”. The authors are grateful to the personnel from Kompetenzzentrum Obstbau Bodensee Center in Germany and to the Non-Governmental Organization Idea Wild. Thanks to Vicente O. Rodríguez for reviewing and revising the manuscript in English.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Oviedo, R.; González, L. Lista nacional de plantas invasoras y potencialmente invasoras en la república de Cuba. Bissea 2015, 9, 1-88. Available from: https://revistas.uh.cu/bissea/article/view/5234.
2. Rai, P. K.; Lee, S. S.; Bhardwaj, N.; Kim, K. H. The environmental, socio-economic, and health effects of invasive alien plants: Review on Tithonia diversifolia (Hemsl.) A. Gray in Asteraceae. S. Afr. J. Bot. 2023, 162, 461-480. Available from: https://doi.org/10.1016/j.sajb.2023.09.038.
3. Vargas, J.K; Zambrano, J.D. Evaluación de la actividad antibacteriana de extractos alcohólicos de Eichornia crassipes frente a Staphylococcus aureus, Salmonella typhi, Candida albicans y Streptococcus pneumoniae, Tesis Doctoral, Universidad de Guayaquil, Facultad de Ciencias Químicas, Ecuador, 2020, 71 pp. Available from: https://repositorio.ug.edu.ec/.
4. López, E.N.; Álvarez, R.; Téllez, A.; Aguayo, J.; Tovar, X. Análisis químico-proximal, fitoquímico y potencial bacteriostático de Eichhornia crassipes. Biotec. 2022, 24, 36-44. Available from: https://www.scielo.org.mx/.
5. Rodríguez, C.N.; Zarate, A.G.; Sánchez, L.C. Actividad antimicrobiana de cuatro variedades de plantas frente a patógenos de importancia clínica en Colombia. Nova 2017, 15, 119-129. Available from: http://www.scielo.org.co/.
6. Khalid, S.; Shaheen, S.; Hussain, K.; Shahid, M.; Sarwar, S. Pharmacological analysis of obnoxious water weed: Eichhornia crassipes (Mart.) Solms. J. Anim. Plant. Sci. 2020, 30,1465-1475. Available from: https://www.researchgate.net/.
7. Ratnani, R. D.; Arianti, F. D.; Sasongko, N. A. Exploring the potential of water hyacinth weed (Pontederia crassipes) as an environmentally friendly antifungal to realize sustainable development in lakes: A review. Case Studies in Chemical and Environmental Engineering. 2024. 100702. Available from: https://doi.org/10.1016/j.cscee.2024.100702.
8. Tulika, T.; Mala, A. Pharmaceutical Potential of Aquatic Plant Pistia stratiotes (L.) and Eichhornia crassipes. J. Plant. Sci. Special Issue: Medicinal Plants 2015, 3,10-18. Available from: https://www.researchgate.net/profile/.
9. Shanab, M.M.; Shalaby, E.A. Biological activities and anticorrosion efficiency of water hyacinth (Eichhornia crassipes). J. Med. Plant. Res. 2012, 6, 3950-3962. Available from: https://academicjournals.org/journal/JMPR/.
10. Gebrehiwot, H.; Dekebo, A.; Annisa, M.E. Chemical Composition, Pharmacological Activities and Biofuel Production of Eichhornia crassipes (Water Hyacinth): A Review. OTCSA 2022, 9, 849-868. Available from: https://dergipark.org.tr/en/download/article-file/2119359.
11. Eden, W.T.; Wahyuono, S.; Cahyono, E.; Astuti, P. Phytochemical, Antioxidant, and Cytotoxic Activity of Water Hyacinth (Eichhornia crassipes) Ethanol Extract. J. Trop. Nat. Prod. Res. 2023, 7, 3606-3612. Available from: https://web.s.ebscohost.com/abstract.
12. Yernazarova, G.I.; Ramazanova, A.A.; Turasheva, S.K.; Almalki, F.A.; Hadda, T.B.; Orazova, S.B.; ... Amangeldinova, M.E. Extraction, Purification and Characterisation of four new alkaloids from the water plant Pistia stratiotes: POM Analyses and Identification of Potential Pharmacophore Sites. Res. J. Pharm. Technol. 2023, 16, 3410-3416. Available from: https://www.rjptonline.org/AbstractView.aspx?PID=2023-16-7-61.
13. Upadhyay, P.; Ali, Z.; Verma, N. A review on Pistia stratiotes L. Adv. Pharm. Toxic. 2023, 24, 29-38. Available from: https://web.s.ebscohost.com/abstract.
14. Gupta, V.; Tyagi, S.; Jain, P.; Tripathi, R. Evaluation of the Bioactive compounds and its Functional role in the Aquatic weed Pistia stratiotes. Res. J. Pharm. Technol. 2024. 171, 87-95. Available from: https://10.52711/0974-360X.2024.00014.
15. Ghosh, S.K. Efficacy of plant based formulation against yellow mite of chilli (Polyphagotarsonemus latus Banks). Int. J. Trop. Insect. Sci. 2023, 43, 645-654. Available from: https://link.springer.com/article/10.1007/s42690-023-00967-y.
16. Plasencia, J.M. Flora acuática de la provincia de Camagüey, Cuba. Polibotánica 2008, 25, 17-28. Available from: https://www.scielo.org.mx/pdf/polib/n25/n25a3.pdf.
17. Lima, L. Niveles de plomo, zinc, cadmio y cobre en el Río Almendares, Ciudad Habana, Cuba. Rev. Int. Contam. Ambient. 2005, 21,115-124. Available from: https://www.scielo.org.mx/pdf/rica/v21n3/0188-4999-rica-21-03-115.pdf.
18. González, S.G. La colección de plantas acuáticas del Jardín Botánico Nacional de Cuba. Rev. Jard. Bot. Nac. 2009, 30, 15-20. Available from: https://www.jstor.org/stable/42597377.
19. Duarte, N.S.P.; Arroyo, N.C.; Herrera, K.C.; Duarte, M.R.P. Co-digestión del purín de cerdo y la Eichornia crassipes: alternativa para el manejo de estos residuos en Cuba. Hig. San. Amb. 2019, 19, 1765-1774. Available from: https://saludpublica.ugr.es/sites/dpto/spublica/public/inline-files/bc5d640023ef1d4.
20. Seisdedo, M.; Díaz, M.; Barcia, S.; Arencibia, G. Análisis comparativo de la calidad del agua de dos embalses de la cuenca Arimao, Cuba (2014-2015). Rev. Cub. Invest. Pesq. 2017, 34, 60-67. Available from: https://aquadocs.org/bitstream/handle/1834/12523/60-67.
21. Coetzee, A.J.; Hill, P.M.; Ruiz, T.; Starfinger, U.; Brunel, S. Monographs on invasive plants in Europe N° 2: Eichhornia crassipes (Mart.) Solms. Bot. Let. 2017, 164, 303-326. Available from: https://www.tandfonline.com/doi/full/10.1080/23818107.2017.1381041.
22. Rivero, C. L.; Quiñones-Gálvez, J.; Martínez, A. T. P.; Ortiz, C. C. C.; Paneca, M. R.; Valdéz, G. A. C., Pérez-Gómez, L.; La Rosa, S.; Capdesuñ Ruiz, Y. K. C. Obtención de extractos fenólicos foliares de Moringa oleifera Lam mediante el uso de diferentes métodos de extracción. Biotec. Veg. 2018, 18, 47-56. Available from: https://revista.ibp.co.cu/index.php/BV/article/view/575.
23. Han, Y.S.; Van der Heijden, R.; Verpoorte R. Biosynthesis of anthraquinones in cell cultures of the Rubiaceae. Plant Cell, Tissue and Organ Culture 2001, 67, 201-220. Available from: https://www.researchgate.net/profile/Rob-Heijden/publication/225230138.
24. Schulte, U.; El-Shagi, H.; Zenk, M. H. Optimization of 19 Rubiaceae species in cell culture for the production of anthraquinones. Plant Cell Reports. 1984, 3, 51-54. Available from: https://link.springer.com/article/10.1007/BF00270970.
25. Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of polyphenolies and their antioxidant capacity in fresh plums. J. Agricul. Food Chem. 2003, 51: 6509-6515. Available from: https://pubs.acs.org/doi/abs/10.1021/jf0343074.
26. Oksanen, J.; Blanchet, J.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R package version 2.2-0. 2014. Available from: https://cir.nii.ac.jp/crid/1570291225091856896.
27. Pelegrín-Morales, E.; Siam-Lahera, C.; Arencibia Carballo, G.; Alvarez-Capote, J. S. Valoración de la Bahía de Cabañas para el cultivo de pepino de mar Isostichopus badionotus en Cuba. Rev. electrón. Vet. 2009, 10, 9pp. Available from: https://aquadocs.org/bitstream/handle/1834/3532/.
28. Peña, O.S.; Rubalcaba, S.C.; Novo, M.F.; Rodríguez, Y.H.; Pérez, A. Evaluación físico-química y microbiológica del agua de la presa El Cacao (Cotorro, Cuba). Hig. San. Amb. 2006, 6, 202-206. Available from: https://saludpublica.ugr.es/sites/dpto/spublica/public/inline-files/.
29. Rodríguez-Tito, J.C.; Pérez, R.M.; Gómez, L.M.; Álvarez, I. Evaluación químico analítica y microbiológica de los embalses Chalons y Parada de Santiago de Cuba. Rev. Cub. Quím. 2017, 29, 418-435. Available from: http://scielo.sld.cu/scielo.php?pid=S2224-54212017000300007&script=sci_arttext&tlng=pt.
30. Juárez, M.L. Identificación de metabolitos secundarios de Eichhornia crassipes (Jacinto de Agua) del Rio Chira, Sullana. Tesis para obtener el título de Químico Farmacéutico. Universidad San Pedro. Facultad de Medicina Humana. Escuela Profesional de Farmacia y Bioquímica. Perú. 2019, 50 pp. Available from: http://publicaciones.usanpedro.edu.pe/bitstream/handle/USANPEDRO/9260/Tesis_60128.
31. Tyagi, T.; Agarwal, M. Phytochemical screening and GC-MS analysis of bioactive constituents in the ethanolic extract of Pistia stratiotes L. and Eichhornia crassipes (Mart.) Solms. J. Pharm. Phytoch. 2017, 6,195-206. Available from: https://www.phytojournal.com/archives/2017/vol6issue1/PartC/6-1-11-956.pdf.
32. Tyagi, T. Phytochemical screening of active metabolites present in Eichhornia crassipes (Mart.) Solms and Pistia stratiotes (L.): Role in ethanomedicine. J. Pharm. Educ. Res. 2017, 6, 40-56. Available from: http://www.ajper.com/admin/assets/article_issue/1508170696.pdf.
33. Sudirman, S.; Aprilia, E.; Janna, M. Kandungan Senyawa Polifenol dan Aktivitas Antioksidan Daun Tumbuhan Apu-apu (Pistia stratiotes) dengan Metode Pengeringan yang Berbeda. J. Peng. Hasil Perik. Ind. 2022, 25, 235-243. Available from: https://journal.ipb.ac.id/index.php/jphpi/article/download/41523/23924.
34. Tovar, X.; Favela, E.; Volke, T.L.; Escalante, E.; Díaz, I.J.; Córdova, J.A.; Téllez, A. Influence of the geographical area and morphological part of the water hyacinth on its chemical composition. Ing. Agríc. Biosist. 2019, 11, 39-52. Available from: https://www.scielo.org.mx/pdf/inagbi/v11n1/2007-4026-inagbi-11-01-39-es.pdf.
35. Shanab, M.M.S.; Shalaby, A.E.; Lightfoot, A.D.; El-Shemy, A.H. Allelopathic Effects of Water Hyacinth (Eichhornia crassipes). 2010. Plos ONE, 5: e13200. Available from: https://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0013200&type=printable.
36. Souza, C.T.A. Identificação de antocianinas em raízes de Eichhornia crassipes (Mart.) Solms. Tesis de grado, Universidade Estadual Paulista “Júlio de Mesquita Filho” – UNESP, Faculdade de Ciências Farmacêuticas, Araraquara, Brasil. 2017, 80 pp. Available from: https://repositorio.unesp.br/server/api/core/bitstreams/8e57abda-98c8-4171-bc6f-88eb61e255c5/content.
37. Rufchaei, R.; Abbas-Mohammadi, M.; Mirzajani, A.; Nedaei, S. Evaluation of the Chemical Compounds and Antioxidant and Antimicrobial Activities of the Leaves of Eichhornia Crassipes (Water Hyacinth). Jundishapur J. Nat. Pharm. Prod. 2022, 17, e101436.
Available from: https://brieflands.com/articles/jjnpp-101436.pdf.
38. Herrera, R. S.; Verdecia, D. M.; Ramírez, J. L. Chemical composition, secondary and primary metabolites of Tithonia diversifolia related to climate. Cuban J. Agric. Sci. 2020. 54, 425-433. Available from: https://www.redalyc.org/articulo.oa?id=653767640013.
39. Prajitha, T.; Bai, R. S. Evaluation of heterotoxicity and identification of allelochemicals of leaf extract of invasive Senna spectabilis (DC) HS Irwin and Barneby. Plant. Ecol. 2024. 225, 285-299. Available from: https://doi.org/10.1007/s11258-024-01397-7.
Received: April 14, 2024 / Accepted: July 18, 2024 / Published: 15 September 2024
Citation: Citation: Hernández-Fernández L, Linares-Rivero C, Quiñones-Galvez Y, Lorenzo-Feijoo JC, Acosta Y, González-De Zayas R. Promising organic compounds in invasive aquatic plants identified in freshwater lagoons in Cuba. Bionatura journal. 2024;1(3):15. doi: 10.70099/BJ/2024.01.03.15
Additional information Correspondence should be addressed to coraleslhf@gmail.com
Peer review information. Bionatura thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
ISSN.3020-7886
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Publisher's Note: Bionatura Journal stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2024 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).