Effect of Five Concentrations of Aqueous Extracts of Pleurotus ostreatus P. Kumm and Tagetes minuta L. on the Mortality of Two Nematodes in a Laboratory Setting

Madison Chango1, Gabriela Rosero2,*, Norma Erazo1,2, Pablo Álvarez1,3
1 Facultad de Recursos Naturales, Escuela Superior Politécnica de Chimborazo (ESPOCH) / Riobamba / Ecuador; madison.chango@espoch.edu.ec.
2 Grupo de Investigación y Desarrollo para el Ambiente y el Cambio Climático GIDAC-ESPOCH / Riobamba / Chimborazo / Ecuador; nerazo@espoch.edu.ec.
3 Grupo de Estudios Fitoentomológicos, Escuela Superior Politécnica de Chimborazo (ESPOCH) / Riobamba / Ecuador; pablo.alvarez@espoch.edu.ec.
* Correspondence: gabriela.rosero@espoch.edu.ec; Tel.: +593 999184660
Available from. http://dx.doi.org/10.21931/BJ/2024.01.01.9
ABSTRACT
The nematode attacks affect several plant species of Ecuadorian crops. There are fungi and plants with nematicide ability that have agricultural interest. This study assessed the effect of five concentrations of aqueous extracts of Pleurotus ostreatus and Tagetes minuta on the mortality of Meloidogyne spp. and Panagrellus redivivus nematodes in a laboratory setting. The aqueous extracts were obtained through hydro distillation where concentrations of 0%, 0.5%, 5%, 25%, 50% and 100% were prepared. A wholly randomized single-factor design was used for the P. ostreatus extract and a bifactorial for the T. minuta extract (leaves and flowers). The number of dead individuals was evaluated, and the efficacy and LC50 were determined. T. minuta leaf extract showcased higher nematicide activity against P. redivivus with an LC50 of 8.03 ppm; when applied to Meloidogyne sp., the extract showed nematicide activity with an LC50 of 0.01 ppm. For P. ostreatus extract, the greatest nematicide activity against P. redivivus was an LC50 of 1.22 ppm and nematicide activity against Meloidogyne sp., was an LC50 of 0.01 ppm. The aqueous extract of T. minuta flowers showed low nematicide activity and the aqueous extract of T. minuta leaf showed the best nematicide activity.
Keywords: nematicide; Tagetes minuta; Pleurotus ostreatus; Panagrellus redivivus; Meloidogyne sp.
INTRODUCTION
Nematodes are one of the phytopathogens that cause significant losses in global agricultural production, with estimated losses of up to one hundred billion dollars annually1. This group of phytoparasites is characterized by its diversity, complexity, and wide distribution across all productive agroecosystems worldwide2. These qualities and peculiarities enable them to induce other diseases3, potentially causing annual losses between 11 and 14%, or even higher if another pathogen is introduced4.
Ecuador has not been exempt from nematode attacks; reports from Ecuadorian fields indicate the presence of: Meloidogyne incognita, Meloidogyne javanica, Meloidogyne arenaria, Meloidogyne hapla, Meloidogyne graminicola, Rotylenchulus reniformis, and Nacobbus aberrans5. These nematodes affect plant species that are part of the daily diet of every Ecuadorian, as well as those destined for export, such as watermelon, melon, cucumber, sugarcane, corn, tomato, onion, pineapple, papaya, passion fruit, rice, summer flowers, etc.
Given this situation, one nematode control alternative has been synthetic chemical nematicides such as carbamates and organophosphates. However, these have shown alarming side effects, high toxicity, residue persistence, and development of resistance6. Therefore, for several years now, there has been a search for more benign control methods for the environment and humans 7.
Various research projects are currently being conducted for the biological control of different phytopathogens1,4,8. One of these alternatives is the use of plant extracts9,10,11, which are characterized by their biological origin, biodegradability, and minimal negative impact on human health and the environment. These extracts have shown the ability to act through their metabolites and exhibit nematicidal12,13,14, insecticidal, acaricidal, or herbicidal activity. In this research, we worked with aqueous extracts of Tagetes minuta and Pleurotus ostreatus.
One of the extracts that have shown promising results as nematicide is garlic bulbil extract, which reduced galling index by 73%, egg and juvenile production by 80%, and female population by 94%15. A reality close to our area regarding integrating a biological control alternative has been the use of the Tagetes genus, which has pesticide characteristics related to its allelopathic function13.
Pleurotus ostreatus is a fungus native to China but has been distributed worldwide except for the Arctic11. This fungus is known as oyster mushroom and is considered a health promoter and environmental restorer16. Tagetes minuta is an annual aromatic herb that grows in temperate grasslands and mountainous regions of South America8; it is known for being arthropod repellent and having herbicidal, nematicidal, insecticidal, fungicidal, antiviral properties, etc. According to research carried out, these particularities are due to its components: ocimenones (Z) and (E), piperitone, piperitenone, limonene, tagetone and caryophyllene17. Dihydrotagetone inhibits the hatching of Meloidogyne incognita eggs by 72 to 79% in 14 days; in the case of juveniles (Z)-β-ocimene is lethal in 72 hours18.
Knowing the work with plant extracts in essential oils, which require complex processes to obtain, this research used the aqueous extract, which is the unstable liquid emulsion of saturated vapor and essential oil10 of Tagetes minuta and Pleurotus ostreatus to evaluate the nematicide effect of the aqueous extracts of Pleurotus ostreatus and Tagetes minuta in the laboratory and to determine the best LC50 of Pleurotus ostreatus and Tagetes minuta on Meloidogyne spp. and Panagrellus redivivus in the laboratory.
Panagrellus redivivus is a free-living nematode that lives in humid environments in a state of fermentation and can feed on various cereals. Thanks to its qualities, tiny size, rapid growth, short life cycle, high fecundity, and easy handling, it is one of the most used nematodes in research work19. For its part, Meloidogyne spp. is an endoparasitic phytonematode that completely penetrates the root to feed, develop and reproduce through eggs20, it is characterized by causing galls, damaging the root system and causing dwarfism, chlorosis, wilting, defoliation or premature senescencese2.
MATERIALS AND METHODS
T. minuta was collected in the community San José de Cunduana, Licán Canton, Province of Chimborazo (Longitude 1°37'40 "S, Latitude 78°43'17''W, Altitude 3085 masl). 0.5 kg of leaves and 1 kg of fresh flowers were collected from native crops; they were cut into pieces of 1 cm long, placed in ziploc bags (17.7cm x 19.5cm) separately and transported in a flex-foam thermal cooler (Century-T40-3).
P. ostreatus and P. redivivus were cultured and provided by the store of the project "Study of the diversity of nematophagous fungi associated with the rhizosphere of tomato (Solanum lycopersicum L.) in three locations in the Province of Chimborazo", executed during 2018 and 2019 at the Biological Sciences Laboratory. P. redivivus was raised in a nutrient medium of oats in 250 g per 200 ml of sterile distilled water. After 15 days, an aliquot of the P. redivivus culture was taken with a flat-tip brush (0 Ø) and placed in a 100 ml glass bottle with sterile distilled water, this process was repeated until the suspension was opaque. Next, a 10 ml sample was placed in petri dishes (90 mm Ø) and 50 nematodes were fished for each of the experimental units with the help of round-tipped brushes (0 Ø - 4/0 Ø) (Figure 1).
Figure 1. Nematode fishing brushes (A), Culture of P. redivivus (B), Inoculation of P. redivivus (C), Suspension of P. redivivus (D).
Both plant materials were processed in the Laboratory of Pharmaceutical Technology of the Faculty of Sciences at ESPOCH, Riobamba Canton, Chimborazo Province (01°38'51 ''S, 78°40'59''W, altitude: 2850 masl)., the hydro-distillation method used by Silva21 was applied: in a two 500 ml mouths, the chopped plant material was placed with hot water up to 3/4 parts of the funnel's capacity, in such a way that all the material was submerged, the heat was applied to start boiling and steam dragging, obtaining; as a result the aqueous extract. Once the aqueous extracts were received, the concentrations were prepared at 0%, 0.5%, 5%, 25%, 50% and 100% with sterile distilled water, each in 50 ml screw-top glass bottles containing. They were stored in a refrigerator (LG- Side by side of 615 l) at 5°C.
Meloidogyne sp. was obtained from infected root samples with nodules in greenhouse plantations of red bell pepper (Capsicum annuum) around 4 months old, located in the sector Quillan Loma Alto, Izamba Parish, Tungurahua Province (01°13'11''S, 78°33 '38''W, altitude 2672 masl). The root with nodules was placed in ziploc bags (17.7cm x 19.5cm) and transported in a flex-foam thermal cooler (Century-T40-3). The tray method for the extraction of Coyne and Claudios22 nematodes was used as a principle with the following adaptations: The roots were washed to remove the adhering soil and cut into 1 cm long segments, 10 g of roots were placed on a napkin (23cm x 24cm) folded in 4 parts, later a tie was made, forming a kind of tea bags, which were suspended in a 500 ml glass jar with saline solution at 0.9% during 24 hours (Figure 2D).
After 24 hours, juvenile 2 (J2) was briefly identified using a stereoscope (Motic SMZ-171). The morphological characteristics detailed by Jaraba, Lozano and Suárez23 and the illustrations by Carmona and Padilla24 were utilized as a guide. It was also considered that when working with the females of Meloidogyne sp. the result was youth 2 (J2). Finally, 10 ml of the 0.9% saline solution was taken. The tea bag with nodules was suspended in glass petri dishes (90 mm Ø) and 20 nematodes were fished for each experimental unit with a traditional fishing instrument (Figure 2A).

Figure 2. Nematodes fishers (A), Pepper crop with nodules. (B), Root nodules with Meloidogyne (C), Adapted method of Coyne and Claudius (D).
Bioassays were carried out in the Biological Sciences Laboratory of the Faculty of Natural Resources at ESPOCH. The number of juveniles 2 (J2) of the nematodes P. redivivus and Meloidogyne sp were obtained. For each of the experimental units, 6 ml of the different concentrations prepared (0%, 0.5%, 5%, 25%, 50% and 100%) of the aqueous extracts of T. minuta leaf-flower and P. ostreatus, was placed in each of the sections of the plastic tripetri boxes (90 mm Ø) according to the treatment. Each experimental unit counted the number of dead and alive J2 nematodes with the help of the stereoscope. Data were recorded every 4 h during the day, and the following efficacy equation of Abott25 was applied.

Where:
IT= Live nematodes in the control
it= Live nematodes in the treatment
The Lethal Concentration 50 (LC50) was estimated through a regression based on the efficacy of the different concentrations of the aqueous extracts of T. minuta and P. ostreatus on the mortality of the populations of P. redivivus and Meloidogyne sp. The LC50 was determined using the EC50 Estimator library of the R programming language of the RStudio version 2022.07.2 program.
The data analysis was performed for each exposure time, for which two types of experimental designs were used: A bifactorial complete randomized design for the aqueous extracts of T. minuta leaf and flowers and a completely randomized single factor for the aqueous extract of P. ostreatus, in both cases with five concentrations (0%, 0.5%, 5%, 25%, 50% and 100%) and two nematodes (Panagrellus redivivus and Meloidogyne sp.).
RESULTS
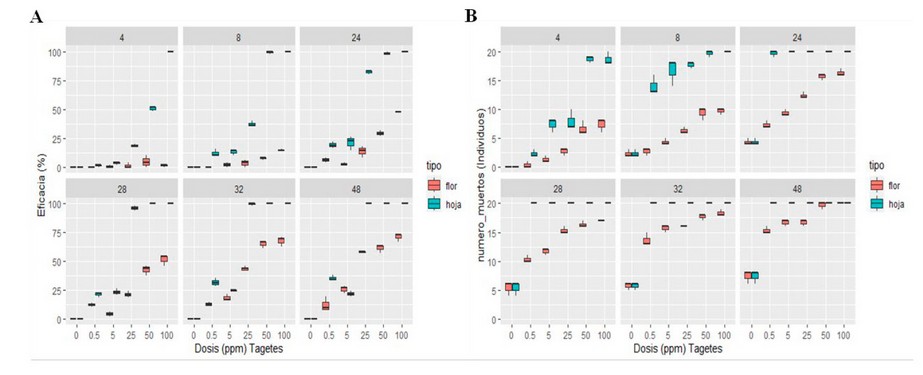
Figure 3. Aqueous extract of Tagetes minuta leaf and flower against Panagrellus redivivus (A), Aqueous extract of Tagetes minuta leaf and flower against Meloidogyne sp. (B).
Figure 3 (A) shows the efficacy of the aqueous extract of T. minuta leaf and flower on the mortality of the nematode P. redivivus during 48 h of exposure. The efficacy of the aqueous extract of T. minuta leaf was higher than that of flowers; this reached an efficacy in mortality more significant than 50% of the population of P. redivivus from the first 4 hours of exposure in concentrations of 50% and 100% whose efficacy was 50.57% and 100% respectively. Finally, after 48h of exposure, it reached an efficacy of 100% in the concentrations of 25%, 50% and 100%. On the other hand, the aqueous extract of T. minuta flower in none of the concentrations managed to reach an efficacy of 100% in the mortality of P. redivivus; the maximum efficacy reached was 71% in the 100% concentration.
Figure 3 (B) shows the efficacy of the aqueous extract of T. minuta leaf and flower on the mortality of the nematode Meloidogyne sp. during 48 h of exposure, showing that the efficacy of the aqueous extract T. minuta leaf was superior to that of flowers, this reached an efficacy more significant than 50% in the mortality of the population of Meloidogyne sp. from the first 4h of exposure in the 50% and 100% concentrations with the efficacy of 93.33% in both cases and finally reached an efficacy of 100% after 48h of exposure in the concentrations of 0.5%, 5%, 25%, 50% and 100%. The aqueous extract of T. minuta flower reached an efficacy of 100% in the highest concentration at 100%.
The results of the mortality caused by P. redivivus and Meloidogyne sp. by the aqueous extracts of T. minuta leaf and flower showed through an analysis of variance at 48 h of exposure that in both cases the factors analyzed concentration (0%, 0.5%, 5%, 25%, 50%, 100%) and type (flower-leaf) were highly significant, therefore with the Tukey Test it was concluded that for the P. redivivus nematode the best concentrations were: 100%, 50% and 25%; and for the nematode Meloidogyne sp. the best concentrations were 100% and 50%; in both cases the best type of extract coincided with the aqueous extract of T. minuta leaf.
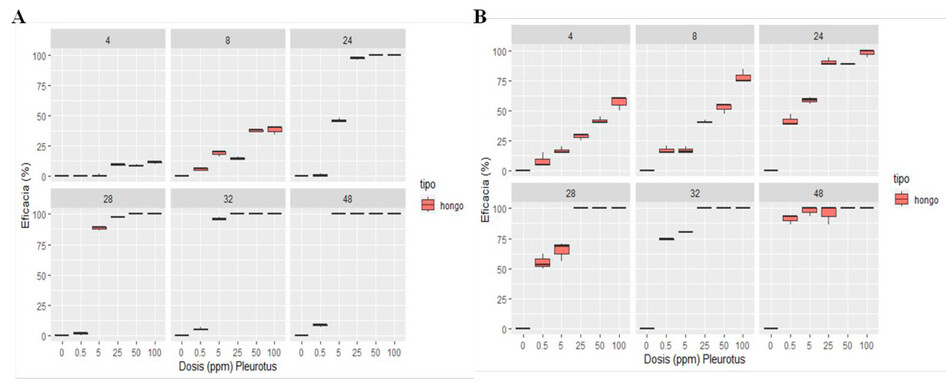
Figure 4. Aqueous extract of Pleurotus ostreatus against Panagrellus redivivus (A), Aqueous extract of Pleurotus ostreatus against Meloidogyne sp. (B).
Figure 4 (A) shows the efficacy of the aqueous extract of P. ostreatus on the mortality of the P. redivivus nematode during 48 h of exposure, showing that the effectiveness of said extract was greater than 50% at 24 h of exposure in concentrations at 25%, 50% and 100% with efficacy of 97.09%, 100%, 100% respectively and at 48h the efficacy of the aqueous extract on the mortality of P. redivivus was 100% in the concentrations at 5%, 25%, 50% and 100%.
Figure 4 (B) shows the efficacy of the aqueous extract of P. ostreatus on the mortality of the Meloidogyne sp. nematode during 48 h of exposure; this indicates that the extract presented an efficacy more significant than 50% at 4h of exposure in the 100% concentration with an efficacy value of 56.67% and at 48h the concentrations at 0.5%, 5%, 25%, 50% and 100% achieved an efficacy of 91.11%, 97.78%, 100% and 100% respectively.
The results of the mortality caused to P. redivivus and Meloidogyne sp. by the aqueous extract of P. ostreatus showed through an Analysis of Variance at 48 h of exposure that in both cases the factor analyzed concentration (0%, 0.5%, 5%, 25%, 50%, 100%) was highly Significant therefore with the Tukey test it was concluded that the best concentrations for both cases were: 100%, 50%, 25% and 5%.
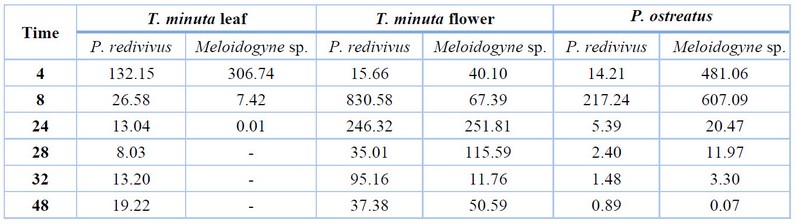
Table 1. LC50 of the aqueous extract of Tagetes minuta and Pleurotus ostreatus against nematodes.
According to treatments against P. redivivus, Table 1 shows that the lowest aqueous extract of T. minuta leaf was 8.03 ppm at 28h of exposure with a minimum limit of - 4.03 ppm and a maximum of 20.10 ppm, while in the case of the aqueous flower extracts the lowest LC50 was 15.66 ppm after 4 hours of exposure with a minimum limit of -112.42 ppm and a maximum of 143.74ppm.
The highest LC50 in the aqueous extract of the leaf was 132.15 ppm in the first 4 hours of exposure with a minimum limit of -160.14 ppm and a maximum of 424.43 ppm; on the other hand, the aqueous extract flower was 830.58 ppm after 8 hours of exposure with a minimum limit of -5929.97 ppm and a maximum of 7591.14 ppm.
The lowest LC50 of P. ostreatus aqueous extract was 0.89 ppm at 48h of exposure, with a minimum limit of 0.84 ppm and a maximum of 0.95 ppm. In comparison, the highest LC50 was 217.24 ppm after 8 hours of exposure with a minimum limit of -2776.20 ppm and a maximum of 3210.68 ppm. In this case, the LC50 decreases as time passes.
According to Meloidogyne sp. results, the lowest aqueous extract of T. minuta leaf was 0.01 ppm at 24h of exposure with a minimum limit of 0.01 ppm and a maximum of 0.02 ppm. In contrast, in the aqueous flower extracts, the lowest LC50 was 11.76 ppm after 32h of exposure, with a minimum limit of -194.39 ppm and a maximum of 217.91 ppm.
The highest LC50 in the aqueous extract of the leaf was 306.74 ppm in the first 4h of exposure with a minimum limit of -4633.86 ppm and a maximum of 5247.33 ppm; on the other hand, the aqueous extract of flowers was 251.81 ppm after 24 hours of exposure with a minimum limit of -1972.92 ppm and a maximum of 2476.54 ppm. In the case of the aqueous extract of the leaf, it reaches its LC50 in just 24 hours of exposure.
The lowest LC50 of P. ostreatus aqueous extract was 0.07 ppm at 48h of exposure, with a minimum limit of 0.02 ppm and a maximum of 0.11 ppm. In comparison, the highest LC50 was 607.09 ppm after 8 hours of exposure with a minimum limit of -3935.96 ppm and a maximum of 5150.14 ppm; in this case, the LC50 decreases as time passes.
DISCUSSION
The aqueous extract of T. minita leaf presented an efficacy of more than 50% in 48 h of exposure in concentrations of 25%, 50% and 100% against P. redivivus (Figure 1) and in concentrations of 0.5%, 5%, 25%, 50% and 100% in the case of Meloidogyne sp. (Figure 1). This result is similar to that of Murga26, where the aqueous extract of T. minuta was used to control Meloidogyne incognita in paprika pepper. They concluded that T. minuta leaf could limit root nodulation by eliminating infective J2 juveniles up to 50%. In the same way, Iannacone27 was observed to it cause a mortality more significant than 50% in eggs and J2 juveniles.
The efficacy to a greater or lesser degree of the aqueous extracts of Tagetes is directly associated to the presence of secondary metabolites, according to the evaluations made by Zygadlo28, the aqueous extract of T. minuta leaves present dihydroxyacetone, (E)-tagetone and limonene; instead, the extract of flowers only have (E)-tagetone, a reason that could explain the low efficacy of the flowers aqueous extract. However, these metabolites are toxic to specific organisms and microorganisms18; therefore, toxicity depends on the percentage in which it is present, and this, in turn, relies on the geographical location and abiotic factors such as high and low temperatures, drought, alkalinity, salinity, UV light, etc29.
Aqueous or oily extracts that have terpenes with phenolic, hydroxyl, or carboxylic groups have been characterized by having greater nematicide activity14; the genus Tagetes presents these groups and in the case of T. minuta it gives the group of terpenes. These same metabolites have a mechanism of action that increases lipid peroxidation rates, causing an induction of oxidative stress30.
The aqueous extract of T. minuta leaf presented the best results with both P. redivivus and Meloidogyne sp. due to its secondary metabolites. Dihydrotagetone and (E)-tagetone have been the most found in investigations related to the nematicide action of the genus Tagetes towards nematodes. When comparing the nematicide action of the extract of Tagetes zypaquirensis with a nematicide of chemical origin (carbofuran), the extract showed a similar action, reducing the populations of Meloidogyne spp. Therefore, they highlighted that this extract can become an alternative for managing root nodules13.
Limonene is a metabolite capable of reducing the hatching of Meloidogyne incognita eggs by 72 to 79% in 14 days18. In this case, the essential oil was used, so there was the presence of (Z)-β-ocimene, which was attributed to the mortality of J2 juveniles in 72 hours; possibly, this metabolite is also found in the aqueous extract used and is the explanation for the high mortality rate of nematodes since it contains a minimum percentage of essential oil31. Limonene is one of the metabolites present in the genus Tagetes and stands out for causing the mortality of the J2 of Meloidogyne sp. linked to the percentage in which it is present9.
The best LC50 of the aqueous extract of T. minuta was that of the leaf; for P. redivivus it was 8.03 ppm (0.008 mg/ml) at 28h, and for Meloidogyne sp., it was 0.01 ppm (0.00001 mg/ml) after 24 hours of exposure (Table 1). The results of the LC50 are different from those found by Herrera and Sandoval32, who determined that the best LC50 for Meloidogyne sp. of the ethanolic extract of T. minuta was 0.0017 mg/ml, possibly due to the type of extract handled, geographic location, and abiotic factors29 since the data correspond to plant tissue collected in Peru. Zarate33 determined the LC50 of the essential oil extract of Tagetes lucida, a species of the Tagetes family, which was 0.06 mg/ml, which reduced between 63 and 80% of tomato root galls.
Regarding the exposure time of the LC50, this is related to the time required by the metabolism of the nematode to unfold the secondary metabolites or with the speed of action of the metabolites of the oil inside the nematode34. In this case, it is related to the secondary metabolites present in the aqueous extract. Despite the lack of research on aqueous extracts, the values found for the LC50 of the aqueous extract of T. minuta constitute a valuable reference for the management of plant substances.
The aqueous extract of P. ostreatus presented an efficacy more significant than 50% against P. redivivus (Figure 2) in concentrations of 5%, 25%, 50% and 100%, in the case of Meloidogyne sp. (Figure 2) in concentrations of 25%, 50% and 100% in both cases at 48h of exposure. Similar results show an efficacy in the mortality of 65.2% of Globodera pallida12. In addition, this aqueous extract of P. ostreatus was reported as having the ability to reduce the number of galls caused by Meloidogyne incognita, and they highlighted that this extract could be a promising measure for the control of this type of phytonematodes35.
The efficacy of the aqueous extract of P. ostreatus on the mortality of both nematodes during 48 h of exposure it presented specific peaks of activity, in the case of P. redivivus the aqueous extract had a peak of efficacy at 24 h, while with Meloidogyne sp. it occurred in the first 4 h of exposure, in both cases the efficacy increased until reaching 100%, a similar investigation reported that Pleurotus ostreatus has a peak of activity during the first 4 h to 24 h of exposure12.
P. ostreatus showed a high mortality rate of the nematodes, which is attributed to its toxic characteristics, which are typical of the genus Pleurotus sp. known to present several species with nematophagous activity, this activity is manifested through the system of production of immobilizing toxin reserves that they present in toxocysts that are produced laterally on the hyphae36. In the same way, Armas37 identified these toxins, finding in the case of P. ostreatus trans-2-decenedioic acid that corresponds to the nematotoxin called NRRL 352638.
The high mortality rate is due to the nematophagous activity that P. ostreatus presents, which begins when the nematotoxin present in the aqueous extract comes into contact with the nematode and immobilizes it, quickly digests it, producing hyphae that grow chemotropically and invade the oral cavity, the anus and the cuticle of the nematode38,39. The best LC50 of the aqueous extract of P. ostreatus for P. redivivus was 0.89 ppm and for Meloidogyne sp. 0.07 ppm in both cases after 48 hours of exposure (Table 1).
After the review of the research that has been carried out on this subject, all of them have aimed to determine the efficacy of the aqueous extract as a nematicide, but the CL5037,40,41 has not been defined. Despite this, each of the investigations emphasizes the particular mode of action and the efficacy of P. ostreatus, which is related to its ability to secrete trans-2-decenedioic acid and some proteases that have not yet been described42. The extract has a high nematicide efficacy, so it is advisable to take precautions when this type of extract is taken to the field since the effect of the metabolites can change once they interact with other molecules found in the soil43.
CONCLUSIONS
The aqueous extracts of Pleurotus ostreatus and Tagetes minuta leaf and flower showed a nematicide effect on Panagrellus redivivus and Meloidogyne sp. The aqueous extract of Tagetes minuta leaf was better than that of flowers; an efficacy of 100% of the aqueous extract of Tagetes minuta leaf was achieved against Panagrellus redivivus in concentrations of 25%, 50% and 100% for Meloidogyne sp. in concentrations 0.5%, 5%, 25%, 50% and 100% after 48h of exposure. In the case of the aqueous extract Pleurotus ostreatus, an efficacy of 100% was achieved against Panagrellus redivivus in the concentrations 5%, 25%, 50% and 100%, for Meloidogyne sp. in concentrations 0.5%, 25%, 50% and 100% at 48h of exposure.
The best lethal Concentration 50 (LC50) of the aqueous extract of Tagetes minuta leaf and flowers was that of leaves; for Panagrellus redivivus, the LC50 of 8.03 ppm in 28 h of exposure and for Meloidogyne sp., the LC50 of 0.01 ppm in 24 h of exposure. Regarding the aqueous extract of Pleurotus ostreatus, the best LC50 against Panagrellus redivivus was 1.22 ppm in 48 h of exposure and against Meloidogyne sp. 0.01 ppm in 48 h of exposure.
Author Contributions: Madison Chango: collection and analysis of data in the laboratory, presentation of results, discussion and writing of the draft article. Gabriela Rosero: laboratory data collection, review, editing and translation of the article. Norma Erazo: direction of the research project and laboratory methodology. Pablo Álvarez: approach to the experimental design and statistical analysis.
Funding: This research has received funding from Escuela Superior Politécnica de Chimborazo through project grant No. IDIPI-268.
Institutional Review Board Statement: This project has been approved by the Polytechnic Council's resolution No. 542. CP. 2021 and has written authorization from the Bioethics Committee head by Prof. María Viteri, dated on September 8th, 2021.
Acknowledgments: The authors of this research thank the engineer Juan Manzano and the biochemist Diego Vinueza for their collaboration in obtaining the aqueous extracts.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Savary, S. et al. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 3, (2019).
2. Castillo, J. Identificación de especies de Meloidogyne spp. presentes en el municipio de Patzicía, Chimaltenango. (Universidad Rafael Landívar, 2014).
3. Sánchez-Moreno, S. & Talavera, M. Los nematodos como indicadores ambientales en agroecosistemas. Ecosistemas 22, (2013).
4. Pérez-Anzúrez, G. et al. Arthrobotrys musiformis (Orbiliales) Kills Haemonchus contortus Infective Larvae (Trichostronylidae) through Its Predatory Activity and Its Fungal Culture Filtrates. Pathogens 11, (2022).
5. Triviño Gilces, C., Navia Santillán, D. & Velasco Velasco, L. Guía para reconocer daño en raíces y métodos de muestreo y extracción de nemátodos en raíces y suelo. INIAP Boletín Divulgativo No. 433 https://repositorio.iniap.gob.ec/bitstream/41000/3849/1/433.PDF (2013).
6. González Cardona, C. & Aristizabal Loaiza, M. Evaluación de un producto nematicida sobre nematodos fitoparásitos del plátano Dominico Hartón (Musa AAB). Acta Agron. 63, 71–79 (2014).
7. López-Alcántara, R. Nematodos, su implicación en la producción agrícola. ECUADOR ES Calid. Rev. Científica Ecuatoriana 2, 10–11 (2015).
8. Muthee Gakuubi, M., Wanzala, W., Wagacha, J. M. & Dossaji, S. F. Bioactive properties of Tagetes minuta L. (Asteraceae) essential oils: A review. Am. J. Essent. Oils Nat. Prod. 4, 27–36 (2016).
9. Ibrahim, S. K., Traboulsi, A. F. & El-Haj, S. View of Effects of Essential Oils and Plant Extracts on Hatching, Migration and Mortality of Meloidogyne incognita | Phytopathologia Mediterranea. Phytopathol. Mediterr. 45, 238–246 (2006).
10. Licet Mena Valdés, L. et al. Determinación de saponinas y otros metabolitos secundarios en extractos acuosos de Sapindus saponaria L. (jaboncillo). Rev. Cuba. Plantas Med. 20, 106–116 (2015).
11. Piska, K., Ziaja, K. & Muszynska, B. Edible mushroom pleurotus ostreatus (Oyster mushroom) – Its dietary significance and biological activity. Acta Sci. Pol. Hortorum Cultus 16, 151–161 (2017).
12. Arteaga, M. B., Soria, C. A. & Ordoñez, M. E. Determinación del potencial nematicida y nematostático in vitro de Pleurotus ostreatus (Jacq. ex Fr.) sobre larvas J2 de Globodera pallida (Stone). Rev. Ecuat. Med. Cienc. Biol. 41, 45–50 (2020).
13. Álvarez S., D. E., Botina J., J. A., Ortiz C., A. J. & Botina J., L. L. Evaluación nematicida del aceite esencial de Tagetes zypaquirensis en el manejo del nematodo Meloidogyne spp. Rev. Ciencias Agrícolas 33, 22–33 (2016).
14. Abdel-Rahman, F. H., Alaniz, N. M. & Saleh, M. A. Nematicidal activity of terpenoids. http://dx.doi.org/10.1080/03601234.2012.716686 48, 16–22 (2012).
15. Martinotti, M. D., Castellanos, S. J., González, R., Camargo, A. & Fanzone, M. Efecto nematicida de extractos vegetales sobre Meloidogyne incognita Nematicidal effects of extracts of garlic, grape pomace and olive mill waste, on Meloidogyne incognita, on grapevine cv Chardonnay. Rev. la Fac. Ciencias Agrar. 48, 211–224 (2016).
16. Naim, L. et al. Variation of Pleurotus ostreatus (Jacq. Ex Fr.) P. Kumm. (1871) performance subjected to differentdoses and timings of nano-urea. Saudi J. Biol. Sci. 27, 1573–1579 (2020).
17. Cornelius, W. W. & Wycliffe, W. Chapter 90 - Tagetes (Tagetes minuta) Oils. in Essential Oils in Food Preservation, Flavor and Safety (ed. Preedy, V. R.) 791–802 (Academic Press, 2016). doi:https://doi.org/10.1016/B978-0-12-416641-7.00090-0.
18. Singh, P. Management of Plant-parasitic Nematodes by the Use of Botanicals. J. Plant Physiol. Pathol. 02, (2014).
19. de Lara, on et al. La importancia de los nematodos de vida libre.
20. Guzmán-Piedrahita, O. A., Carolina, C. & López-Nicora, H. D. Physiological interactions of plants with plant-parasitic nematodes: A review. Bol. Cient. del Cent. Museos 24, 190–205 (2020).
21. Silva Olivo, J. C. “Evaluación de la actividad insecticida y/o repelente ‘in vivo’ de extracto acuoso de Artemisia absinthium y aceites esenciales de Tagetes minuta y Tagetes zipaquirensis sobre Lasius niger. (Escuela Superior Politécnica de Chimborazo, 2013).
22. Coyne, D. L., Nicol, J. M., Traducción, C.-C. & Verdejo-Lucas, S. Nematología práctica: Una guía de campo y laboratorio. (International Institute of Tropical Agriculture (IITA), 2007).
23. Jaraba, J. D., Lozano, Z. E. & Suárez Padrón, I. E. Meloidogyne incognita (Kofoid and White, 1919) Chitwood 1949 y Meloidogyne arenaria (Neal 1889) Chitwood 1949: Nematodos de las nudosidades radiculares en guayaba (psidium guajava l.) c.V. Manzana en Monteria, Cordoba. Temas Agrar. ISSN-e 0122-7610, Vol. 8, No. 2, 2003, págs. 15-21 8, 15–21 (2003).
24. Carmona, R. & Padilla, W. Morphological, morphometric and molecular identification of Meloidogyne exigua (Göeldi 1887) in coffee (Coffea arabica). Agron. Mesoam. 31, 531–545 (2020).
25. ICA. Manual para la elaboración de protocolos para ensayos de eficacia con PQUA. (Instituto Colombiano Agropecuario, 2020).
26. Murga-Gutiérrez, S. N., Alvarado-Ibáñez, J. C. & Vera-Obando, N. Y. Efecto del follaje de Tagetes minuta sobre la nodulación radicular de Meloidogyne incognita en Capsicum annuum, en invernadero. Rev. peru. biol 19, 257–260 (2012).
27. Iannacone, J. et al. Acute and chronic toxic effect of Tagetes minuta' Black mint' (Asteraceae) and carbaril on six important entomophages in biological control. Biol. 15, 85–97 (2017).
28. Zygadlo, J. A., Lamarque, A. L., Maestri, D. M., Guzman, C. A. & Grosso, N. R. Composition of the Inflorescence Oils of Some Tagetes Species from Argentina. J. Essent. Oil Res. 5, 679–681 (1993).
29. Peralta-Sánchez, M. G. et al. Metabolitos secundarios y clorofilas en cempasúchil en respuesta a estrés salino. Rev. Mex. ciencias agrícolas 5, 1589–1599 (2014).
30. Senatore, F. et al. Antibacterial activity of Tagetes minuta L. (Asteraceae) essential oil with different chemical composition. Flavour Fragr. J. 19, 574–578 (2004).
31. Alejandro Rojas, G. et al. Evaluación in vitro de la actividad nematicida de limoneno, isotiocianato de alilo, eucaliptol, β-citrolenol y azadiractina sobre Meloidogyne incognita (Nematoda, Meloidogynidae). Trop. Subtrop. Agroecosystems 22, (2019).
32. Herrera Moncada, W. L. & Sandoval Fuentes, M. G. Toxicidad del extracto etanólico de plantas de campo y callos in vitro de Tagetes minuta y Tagetes erecta sobre Meloidogyne spp. en Solanum lycopersicum L. Universidad Nacional Pedro Ruiz Gallo (Universidad Nacional Pedro Ruiz Gallo, 2019).
33. Zarate-Escobedo, J. et al. Concentrations and application intervals of the essential oil of Tagetes lucida Cav. against Nacobbus aberrans. Rev. Mex. Ciencias Agrícolas 9,.
34. Mendoza-García, E. et al. Efecto biológico del aceite de Tagetes coronopifolia (Asteraceae) contra Diaphorina citri (Hemiptera: Liviidae). Rev. Colomb. Entomol. 41, 157–162 (2015).
35. Erazo Sandoval, N. S. et al. Effect of Pleurotus ostreatus (Jacq.) and Trichoderma harzianum (Rifai) on Meloidogyne incognita (Kofoid & White) in tomato (Solanum lycopersicum Mill.). Acta Sci. Biol. Sci. 42, (2020).
36. Clémençon, H., Emmett, V. & Emmett, E. E. Cytology and Plectology of the Hymenomycetes. (2012).
37. Armas-Tizapantzi, A. et al. Estructuras tipo toxocistos en Pleurotus ostreatus y P. pulmonarius. Sci. fungorum 49, e1250 (2019).
38. Ernesto, J., El, S., De La, C., Sur, F. & Royse, D. J. La Biología, el cultivo y las propiedades nutricionales y medicinales de las setas Pleurotus spp. Edible mushroom cultivation View project oxidorreductases enzymes View project. (2017).
39. Aguilar Marcelino, L. et al. Los hongos del género Pleurotus como agentes de biocontrol de parásitos de importancia pecuaria. 52, 1375 (2021).
40. Quevedo, A. et al. Interacciones ecológicas de los hongos nematófagos y su potencial uso en cultivos tropicales. Sci. Agropecu. 13, 97–108 (2022).
41. Jansson, H.-B. & Lopez-Llorca, L. V. Hongos nematófagos. 145–173 https://dcmba.ua.es/es/areas/botanica/hongos-nematofagos.html# (2001).
42. Leonardo, H. et al. Activity of the fungus Pleurotus ostreatus and of its proteases on Panagrellus sp. larvae. African J. Biotechnol. 14, 1496–1503 (2015).
43. Arteaga Paredes, M. B. Determinación del potencial nematicida y nematostático in vitro de Pleurotus ostreatus (Agaricales: Pleurotaceae) sobre larvas J2 de Globodera pallida (Tylenchida: Heteroderidae). (Pontificia Universidad Católica del Ecuador, 2018).
Received: October 9th 2023/ Accepted: January 15th 2024 / Published:15 February 2024
Citation: Chango, M.; Rosero, G.; Erazo, N.; Álvarez, P. Effect of Five Concentrations of Aqueous Extracts of Pleurotus ostreatus P. Kumm and Tagetes minuta L. on the Mortality of Two Nematodes in a Laboratory Setting. Bionatura Journal 2024; 1 (1) 9. http://dx.doi.org/10.21931/BJ/2024.01.01.9
Additional information Correspondence should be addressed to gabriela.rosero@espoch.edu.ec
Peer review information. Bionatura Journal thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Publisher's Note: Bionatura Journal stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2024 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
