Phytochemical analysis underlying membrane stabilization and anti-oxidant promising potentials of Acacia nilotica seed extract

Abubakr El-Mahmoudy 1,*
1 Department of Pharmacology, Faculty of Vet. Medicine, Benha University, 13736 Qalioubia, Egypt.
* Correspondence: a.elmahmoudy@fvtm.bu.edu.eg; Tel.: (+20-13-2460640)
Available from. http://dx.doi.org/10.21931/BJ/2024.01.01.31
ABSTRACT
This study has aimed to evaluate the Acacia nilotica seed extract as a natural antioxidant and membrane stabilizer, as well as screening phytochemicals contained in the extract that may underline these activities. Antioxidant activity was evaluated using Phospho-molybdenum and DPPH antioxidant assays; membrane stabilization was evaluated using HRBC-MS assay, whereas phytochemicals were detected using qualitative analytical tests. Acacia nilotica hydro-methanolic extract exhibited potent, concentration (4-512 μg/mL)-dependent antioxidant activity indicated by Phospho-molybdenum and DPPH antioxidant assays, with IC50 values of 100.5 and 33.19 μg/mL, respectively. In addition, the extract exerted membrane stabilizing effects in a concentration (4-512 μg/mL)-dependent manner, with an IC50 value of 41.47 μg/mL. Phytochemical analysis of the plant extract clarified the active biological constituent(s) underlying these effects, including tannins, saponins, gums, flavonoids, anthraquinone glycosides, carbohydrates and proteins. These data may suggest Acacia nilotica extract, according to in vitro assays, as a highly potent antioxidant and membrane stabilizer derived from nature and could be applied as an adjunct therapy in disease conditions associated with inflammation and oxidative stress.
Keywords: Acacia nilotica; antioxidant; membrane stabilizer; anti-inflammatory.
INTRODUCTION
Acacia nilotica (also known as Gum Arabic tree, Egyptian thorn, Prickly Acacia) is a leguminous tree belonging to the Fabaceae family, Plantae Kingdom. It is widely spread in subtropical and tropical countries in Africa and Asia 1. Acacia nilotica is a medium-sized, single-stemmed, thorny tree that may reach up to 25 meters in height and 3 meters in diameter 2.
Acacia nilotica has been used in traditional medicine, and some of its pharmacological activities have been demonstrated. The different types of extracts from different parts of the plant were reported to exhibit anti-aggregatory 3, antihypertensive 4, spasmogenic 4, antifungal 5, anthelmintic 6, antiviral 7, antidiarrheal 8, antibacterial 9, and antimalarial 10 activities. The plant was also tested in vivo for anti-inflammatory 11 and analgesic 12 activities in rats and found positive.
Oxidative stress is a predisposing factor and a direct cause of various disorders in both humans and animals. These disorders may be acute or chronic, for example, diabetes, vascular disease (atherosclerosis), premature aging, decreased immune defense and neuronal degeneration. This fact has drawn the attention of pharmacologists to the crucial role that antioxidants could play in targeting prophylaxis and therapy of related diseases, as well as improving the health status and performance of normal subjects 13. Frequent use of conventional chemical drugs is usually associated with side effects that may be troublesome in chronic conditions. Therefore, searching for a safe alternative to scavenging oxidative free radicals (as hydroxyl (OH·), peroxyl (ROO–), alkoxyl (RO–), and peroxynitrite (ONOO–)) became a need for all concerned personnel, including researchers. Antioxidants scavenge and antagonize active free radicals, most probably before attacking cell membranes and biological systems 14.
The present study, therefore, was adopted to utilize some of the most famous and reliable in vitro assays to evaluate the antioxidant and membrane stabilizing pharmacological properties of a hydromethanolic extract of Acacia nilotica ripe seeds growing around the River Nile and to identify the active principle(s) that might mediate these activities.
MATERIALS AND METHODS
The plant part used
The ripe, dry seeds of Acacia nilotica (Fig. 1) that are commonly used in folk medicine were obtained from our local environment where the seeds are available at shops of herbs and spices elsewhere in Egypt; half kg of the seeds was purchased from a shop at Qalioubia province and confirmed by a Botany specialist.
Chemicals and reagents
DPPH- (2,2-diphenyl-1-picryl-hydrazyl) was obtained from Sigma-Aldrich® Chemical Co. (St. Louis, MO.). All other chemicals/solutions used in the present study were of analytical grade. Reagents used for the detection of different phytochemical groups were prepared as follows:
Mayer's regent: Mercuric chloride (1.36 g) and potassium iodide (5.0 g) were dissolved separately in 60 and 20 mL of water, respectively; both prepared solutions were mixed and completed up to 100 mL with distilled water.
Wagner's reagent: Potassium iodide (2 g) and then Iodine (1.27 g) were dissolved in 5 mL of distilled water, and the solution was completed to 100 mL with distilled water.
Hager's reagent: A saturated picric acid solution is heated, filtered, and cooled.
Dragendorff's reagent: The stock solution was prepared by mixing bismuth sub-nitrate (1.7 g) with distilled water (80 mL) and acetic acid (glacial, 20 mL). Potassium iodide solution (50% w/v, 100 mL) was then added, and the mixture was shaken until dissolved and then kept in a dark bottle. The working reagent was done by premixing 100 ml of the stock solution with 200 ml of glacial acetic acid and making up to the volume of 1 liter with distilled water in a dark bottle.
Tannic acid reagent: Ten g of tannic acid powder in 100 mL of distilled water.
Molisch's reagent: Ten% solution of α-naphthol in alcohol.
Fehling's reagent: Is a mixture (50:50 v/v) of two solutions that should be mixed only upon use; solution-I was prepared by dissolving 6.3 g of copper sulfate-(5H2O) in distilled water containing a few drops of dilute sulfuric acid; solution-II was prepared by dissolving 35 g of potassium tartrate and 15.4 g of NaOH in 100 mL of distilled water.
Benedict's reagent: Prepared by dissolving Sodium citrate (86.5 g) and anhydrous sodium carbonate (50 g) in 400 ml distilled water, then boiling and cooling until clearance. After cooling, copper sulfate-(5H2O) (8.65 g) was dissolved in 75 ml distilled water and added to the clear solution.
Vanillin-hydrochloric acid reagent: Prepared by mixing vanillin (1 g) in alcohol (10 mL) and then adding 10 mL of concentrated hydrochloric acid.
Wilson's reagent: Boric and citric acids in anhydrous acetone.
Millon's reagent: Equal parts of mercury (or mercuric nitrate) and fuming HNO3, diluted with water up to twice the original volume.
Biuret reagent: It consists (per 100 mL final volume) of 0.9 g Sodium potassium tartrate, 0.3 g Copper sulfate.5H2O, and 0.5 g Potassium iodide, all dissolved in order in 40 ml 0.2 M NaOH. Then, the mixture was brought to the final volume (100 mL) by 0.2 M NaOH.
Extraction procedure
The extraction procedure was modified after that adopted by Harborne 15. Plant seeds were refluxed in running tap water and then with distilled water, shade-dried at room temperature and coarsely crushed using a pestle and mortar. After dryness, seed extract was prepared by macerating a weighed amount (250 g) of the crushed seeds in a known volume (2 Litres) of aqueous organic solvent (distilled water: methanol, 70:30, v/v) in covered Erlenmeyer flasks. Maceration was done under refrigeration with continual shaking for 48 hours. The hydro-methanolic extracts were filtered and then concentrated using a water bath with a shaker at 56 °C in clean, pre-weighed glass beakers. The obtained semisolid residues (yields) were weighed and reconstituted in a measured amount of isotonic saline (NaCl 0.85%, w/v). Reconstituted extract concentration was firstly adjusted at 1 mg/mL and then serially diluted in isosaline to get 512, 256, 128, 64, 32, 16, 8 and 4 μg/mL solutions. Appropriate extraction for every phytochemical test was done according to the test applied, as mentioned later. Yield % was calculated as: (Extracted residue weight / Original seed weight) × 100.
Phospho-molybdenum assay
Prieto Pineda 16 stated that the total antioxidant activity can be tested spectrophotometrically by producing the phosphor-molybdenum-(V) complex. The principle of the assay depends on the reduction (decreasing the valency) of Molybdenum-(VI) to Molybdenum-(V) by the action of the unknown test sample and then the production of a greenish phospho-molybdenum-(V) complex in an acidic medium. In a clean, dry test tube, an aliquot (300 μL) of Acacia nilotica seed extract (4 – 512 μg/mL, separately) was mixed with 3 mL of the reagent solution that consists of 0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate. A blank control and standard tubes were prepared by substituting the extract test sample with isosaline and ascorbic acid (2 mM in saline). All tubes were stopped, heated using a shaking water bath at 95°C for ninety minutes, and left to cool at room ambient temperature. After cooling, the amount of absorbance of the mixtures for the blue color was measured at 695 nm against the blank. The antioxidant capacity was estimated concerning the standard (Ascorbic acid 2 mM) using the following equation*:
Antioxidant % = [(A sample ˗ A blank) / (A standard ˗ Ablank)] × 100
*The increase in the degree of the blue color indicates higher antioxidant activity.
DPPH assay
The assay was adopted after Blois 17 and Manzocco, Anese 18. The test principle depends on the fact that the molecule 2,2`-diphenyl-1-picryl-hydrazyl (DPPH-) is categorized as a stable free radical species by the rule of the delocalization of the spare electron over the whole molecule so that the molecule cannot dimerize. Such electron delocalization gives the molecule a deep violet color whenever in methanol or ethanol as the solution, which can be measured spectrophotometrically at a wavelength of 517 nm (absorption). If the DPPH solution is mixed with another solution that donates a hydrogen atom, the reduced form of DPPH is devoid of this violet color. The test was conducted by adding an aliquot (200 μL) of the extract test sample at a particular concentration (4 - 512 μg/mL) to 2 mL of DPPH- solution (0.5 mM in methanol) in a covered dry clean test tube. The blank control and standard tubes were prepared similarly, but the test sample solution was replaced by isosaline and ascorbic acid (2 mM in saline), respectively. After 30 minutes of incubation at room temperature, the absorbance was measured at 517 nm. The percentage of the DPPH- free radical scavenging is calculated using the following equation*:
Antioxidant % = [(Ablank ˗ Asample) / (Ablank ˗ A standard)] × 100
*The decrease in the degree of the violet color indicates higher antioxidant activity
HRBC-MS assay
The membrane stabilizing potential of the hydromethanolic extract of Acacia nilotica seed was evaluated in vitro by HRBC-MS (Human RBC-Membrane stability) assay according to 19 after minor modifications. Membrane stability of RBCs incubated with the extract at serial concentrations (4-512 μg/mL) was tested. A blood sample (10 mL) was collected from a healthy human donor (assured that he has not received any anti-inflammatory drugs within the last two weeks) and added to an equal volume of isosaline (NaCl 0.85% in distilled water, pH 7.4). The fresh blood-saline solution mixture was centrifuged at 900xg for 5 minutes, and the resultant supernatant was carefully pipetted out. The packed cells were re-suspended with an equal volume of isosaline and re-centrifuged. This stepwise was repeated twice until the supernatant became clear. A 10% RBC suspension was then prepared with isosaline and used immediately to investigate the effect of the plant seed extract on RBC membrane stability by challenge with a hypotonic saline solution. A volume of 4.5 mL reaction mixture consisting of 2 mL hypotonic saline (NaCl 0.36 %, pH 7.4), 1 mL of sodium phosphate buffer (0.15 M, pH 7.4), 1 mL of plant extract (4-512 μg/mL) dissolved in isosaline and, finally, 0.5 mL of 10% of the freshly prepared RBC suspension. A standard control was made by including 1.0 mL of diclofenac potassium (100 μg/mL) in isosaline. A blank control tube was prepared similarly by replacing the 1 mL of the test plant extract with 1 mL of distilled water. After that, all prepared tubes were incubated at 37°C for 30 minutes, then centrifugated at 900xg rpm for 5 minutes. The resultant supernatant solutions containing hemoglobin were pipetted into cuvettes, and the absorbances were measured spectrophotometrically at a wavelength of 560 nm.
The percentage membrane-stabilizing capacity was estimated using the following equation*:
% Membrane stability = [(Ablank ˗ Asample) / (Ablank ˗ A standard)] × 100
*Decreasing the degree of red indicates higher membrane stabilizing activity.
Phytochemical analysis
Phytochemical screening of Acacia nilotica for the presence of different active principle groups, including alkaloids, glycosides, anthraquinones, cardiac glycosides, saponins, tannins, phlorotannins, flavonoids, resins, gums, terpenoids, coumarins, oils and proteins was carried out. All tests were performed as triplicates and given marks from (-) to (+++) according to the strength of the color or precipitate that appeared.
Detection of alkaloids
About one g of the crushed seeds was extracted with 10 mL of diluted 1% HCl, with heat aid; then the mixture was filtered 20. In clean and dry test tubes, two mL of the filtrate were treated separately with a few drops of Mayer's, Wagner's, Hager's, Dragendorff's, or tannic acid 10% reagents. Creamy, brown, yellow, deep yellow, and buffy precipitation with those detecting reagents, respectively, was judged as an indicator for an alkaloidal substance content.
Detection of glycosides/carbohydrates
The extraction was done by heating 5 grams of the crushed seeds with 30 mL of distilled water. The watery extract was then decanted, and the supernatant was tested for its content of a carbohydrate and a glycoside substance following the classical procedure reported by 21 and 22, with minor modifications, in the following tests:
Molisch's test: About 0.2 mL of α-naphthol alcoholic solution 10% was added to two mL of the tested water filtrate in a clean and dry test tube; then by the addition of 2 mL of sulphuric acid onto the inside wall of the tube, a bluish violet zone formation denotes presence of glycosides and carbohydrates.
Fehling's test: Equal amounts of the concentrated extract and Fehling's reagent were mixed and heated for a few minutes. Precipitation with a changing of color ranging between yellow to brown indicates the presence of certain glycones as a part (or not) of glycosides and carbohydrates.
Benedict's test: Equal aliquots of the concentrated extract and Benedict's reagent were mixed in a clean and dry test tube and heated for a few minutes. Precipitation, with the change of color to any degree from yellow to red-brown, denotes the presence of reducing sugar(s) as a part (or not) of glycosides and carbohydrates.
To differentiate if the constituent is a glycoside or carbohydrate, Fehling's and Bendedict's tests were repeated twice, the first with an aqueous extract of the seed, while the second with the acidulated (H2SO4) extract (that was then neutralized by 5% NaOH solution); stronger color in the second trial indicates a glycoside in general. Special tests to detect special glycoside categories were performed as follows:
Baljet's test: A few drops of sodium picrate solution were added to 1 mL of the concentrated extract. Orange discoloration denotes the presence of cardiac glycoside(s).
Legal's test: To 100 mg of the crushed seed powder, 2 mL of pyridine solution, a few drops of nitroprusside, and then a few drops of 20% NaOH solution were added successfully. A deep red color indicates cardiac glycoside.
Killer-Killiani test: Two mL of acetic acid (glacial) with a drop of ferric chloride solution was added to five mL (100 mg/mL in methanol) of the crushed seed extract in a clean and dry test tube. One mL of conc. Sulphuric acid was added to form a zone above the prepared mixture. The formation of a bluish-brown ring at the interface indicates the deoxy- -sugar characteristic of cardiac glycosides 23.
Schonteten's Reaction (Borax test): To 2 mL of the aqueous seed extract (1 g/10 mL), 0.1 g of Borax was added and heated until dissolved. A few drops of the liquid were poured into a test tube almost full of water; a green fluorescence indicates anthraquinone glycoside.
Borntrager's test: Half g of the crushed seeds was heated with 6 mL of a diluted acid as 1% HCl or 1% H2SO4 and decanted or filtered. The supernatant/filtrate was then strongly mixed with 5 mL of benzene and then filtered; then, two mL of 10% ammonia solution were added to the supernatant/filtrate. Upon vigorously shaking tube contents, the appearance of a pink, violet, or red coloration in the ammoniacal layer indicates the presence of anthraquinone glycoside(s) 23.
Modified Borntrager's test: To 0.5 gm of the crushed seed powder, 5 mL of 5% solution of ferric chloride and 5 mL dilute HCl were added, and the mixture was heated in boiling water bath for 5 minutes, cooled and shaken gently with an organic solvent as benzene. The organic solvent layer was separated, and an equal volume of dilute ammonia was added. A pinkish-red color in the ammoniacal layer is indicative of anthraquinone glycoside.
Detection of saponins
Foam (Froth) test: The ability of saponin to produce froth upon shaking and emulsion with oil was used for its detection 20. About two g of the crushed seeds were heated in 20 mL of distilled water in a water bath for five minutes and then filtered. Ten mL of the filtrate was mixed with 5 mL of distilled water in a clean, dry 25 cm cylinder and subjected to vigorous shaking to observe froth formation. To complete the test, three drops of olive oil were mixed with the formed froth (if any), shaken vigorously and observed for emulsion formation. At least ten cubic mm height of froth that stands for at least 10 minutes indicates saponin and emulsion formation confirms it.
Hemolysis test: the ability of saponin to reduce surface tension and cause hemolysis was utilized for its detection. Two test tubes were prepared, and 5 mL of 10 % blood suspension in isosaline was placed in each (clear upon centrifugation). 5 mL of isosaline solution was added to one of them, while 5 mL of the seed extract in isosaline was placed in the other tube instead. Both tubes were gently mixed, centrifuged and noticed for hemolysis.
Detection of tannins/phenols
About 2 g of the seed powder was extracted in 20 mL ethanol (50 %) by heating in a water bath for 10 minutes at 70 °C and tested for tannins and other phenolic compounds in the seed extract using the following tests 24.
Gelatin test: Equal amounts of the extract and 1% gelatin solution in sodium chloride (0.85%) were mixed in a clean, dry test tube. The formation of white/cloudy/buffy precipitate indicates the presence of tannins (in general) in the seed extract.
Lead acetate test: Two mL of 10% lead acetate was filtered, and a straightforward solution was added to 2 mL of the extract. A bulky white precipitate indicates the presence of tannin and phenolic compounds.
Phenazone test: A mixture of the seed extract (aqueous) and sodium acid phosphate (0.5 g) was heated, cooled and filtered. When phenazone solution (2%) is added to the filtrate, a bulky colored precipitate is formed if a tannin is there.
Bromine solution test: Equal amounts of the seed extract (aqueous) and bromine solution were mixed. Condensed tannins will give a buff-colored precipitate, while hydrolyzable tannins give no precipitation.
Ferric Chloride test: A few drops of FeCl3 solutions (1%) were added to an aliquot of 2 mL of the prepared extract; the formation of bluish-black or greenish color denotes the presence of gallo- or catechol- -tannins (hydrolyzable or condensed), respectively.
Hydrochloric acid test: Half g of the seed powder was boiled with 5 mL of 1% HCl for 10 minutes; the appearance of a red precipitate refers to phlorotannins 23.
Vanillin test: Two mL of vanillin-HCl reagent were added to an aliquot of five mL of the alcoholic seed powder extract (1 g seed/10 mL alcohol). The formation of a red or pink deposit denotes the presence of gallic acid (a hydrolyzable tannin).
Detection of flavonoids
Shinoda's (Cyanidin) test: Two mL of 10% ethanolic extract of the crushed seed powder (1 g/10 mL; w/v) were mixed with 0.5 ml of HCl (10%) and a few mg of magnesium metal turnings. The development of a reddish color denotes the presence of flavonoids 23.
Wilson's test: Some flavonoids (5-oxyflavones and 5-oxyflavonoles) with Wilson's reagent develop a brightly yellow color with yellowish-green fluorescence if present in the seed extract.
Lead Acetate test: A few drops of clear lead acetate solution (10%) were added to two mL of the crushed seed ethanolic extract in a clean and dry test tube. The appearance of a yellow-colored precipitate denotes the presence of flavonoids.
Alkaline reagent test: Two mL of the seed aqueous extract were treated with 10% ammonium hydroxide solution; yellow fluorescence observation denotes the presence of flavonoids.
Detection of resins
Fifty mL of 95% ethanol was added to about 5 g of the dry grind of the plant seeds. The mixture was heated in a shaking water bath for about 20 minutes and then decanted or filtered. Upon adding about 5 mL of distilled water, a precipitate's formation denotes a resinous content 20.
Detection of Gums/Mucilages:
The extraction was done by dissolving 1 g of the powdered seeds in 10 mL of distilled water in a dry, clean, large test tube. Then, absolute alcohol (25 mL) was added with constant stirring. The appearance of a white/cloudy precipitate denotes the presence of gums/mucilages 25.
Detection of terpenoids/steroids
Presence of terpenoids and derived steroids in Acacia nilotica seeds was carried out by the following tests:
Salkowski's test: One hundred mg of the crushed seeds were extracted in 2 ml of chloroform, and then 3 ml of concentrated H2SO4 were carefully added onto the wall of the test tube. After standing for minutes, reddish coloration at the lower layer confirms the presence of steroids, while turning it into yellow indicates terpenoids 20.
Libermann-Burchard test: The chloroform extract was treated with a few drops of acetic anhydride and then heated. After cooling, an equal amount of concentrated H2SO4 was added carefully onto the inside wall of the test tube. The appearance of a brown ring at the interface and the turning of the upper layer into green indicate the presence of steroids, while the formation of a dark red color indicates terpenoids.
Detection of Fixed oils
Spot (Stain) test: Petroleum ether or benzene seed extracts were tested for fixed oils/fats. A small amount of extract was pressed between the filter paper folds. The appearance of oil stains denotes fixed oil/fat content 26.
Saponification test: A few drops of 0.5 N alcoholic KOH solution were added to a reasonable amount of the seed extract and a drop of ph-ph. The mixture was heated in the water bath for 1~2 h. Soap formation or partial alkali neutralization denotes the presence of fixed oil 26.
Detection of Proteins/Amino acids
A gram of the crushed seed powder was mixed with 10 ml of distilled water in a dry clean test tube and filtered through Whatman No.1 filter paper. Then, the filtrate was subjected to tests for proteins and free amino acids, including:
Millon's test: A few drops of Millon's reagent were added to two mL of the prepared seed filtrate. A buffy white precipitate that turns red upon heating denotes the presence of proteins 27.
Biuret test: An aliquot of 2 mL filtrate was treated with a few drops of Biuret reagent (see above). Turning the light blue color into a violet/mauve color denotes the presence of peptide bonds/proteins 15.
Ninhydrin test: A few drops of 0.2% ninhydrin solution in acetone were added to 2 mL of the seed aqueous filtrate in a clean and dry test tube. A characteristic purple color upon heating indicates the presence of free amino acids 28.
Data presentation and analysis
Data are expressed as mean ± Standard error of the mean of three separate observations (triplicates). Each observation was calculated as % of the activity of the corresponding standard. For in vitro antioxidant assays, the IC50 values of the plant seed extract were calculated for each assay from the logarithms of the used concentration range (4-512 μg/mL). All statistics and graphing procedures were done using the computer program GraphPad Prism® version 6 (CA, USA).
RESULTS
The yield % of the crushed Acacia nilotica seeds when macerated in hydromethanol (70:30, v/v) was 31.4%.
Data from the present study revealed that Acacia nilotica seed hydromethanolic extract exhibited potent total antioxidant activity indicated by phospho-molybdenum assay. The effect was concentration-dependent, being 2.11 ± 0.81 and 35.80 ± 1.35 % at 4 and 512 μg/mL, respectively, with IC50 value of 100.5 μg/mL (Table 1 & Fig. 2).
Concerning DPPH radical-scavenging activity, Table 2 & Fig. 3 show that the DPPH radical-scavenging activity of Acacia nilotica seed extract was concentration-dependent, being 2.56 ± 0.40 and 92.14 ± 0.62 % at 4 and 256 μg/mL, respectively with IC50 value of 33.19 μg/mL.
As indicated by the HRBC-MS assay, Acacia nilotica seed extract exhibited potent membrane stabilizing activity on RBC membranes that was concentration-dependent, being 5.60 ± 2.25 and 89.75 ± 2.83 % at 4 and 256 μg/mL, respectively, with IC50 value of 41.47 μg/mL (Table 3 & Fig. 4).
Phytochemical analysis revealed the presence of tannin-, flavonoid, gum-, anthraquinone glycoside-, saponin-, carbohydrate- and protein-compounds (Table 4). On the other hand, some other phytochemical groups and compounds, including alkaloids, cardiac glycosides, resins, terpenoids, steroids, fixed oils/fats, gallic acid and free aminoacids, were not detected by the used tests (Table 4).
Figure 1. Photograph for the seeds of the Acacia nilotica plant used for extraction.
Table 1. Antioxidant activity (% from 2 mM Ascorbate as a standard) of Acacia nilotica seed extract (4-512 μg/mL) indicated by Phospho-molybdenum assay.
Figure 2. Phospho-molybdenum antioxidant assay. Line graph showing the % of the phospho-molybdenum complex formation by different concentrations of Acacia nilotica seed hydromethanolic extract (4-512 μg/mL); values are means ± SEM of triplicates.
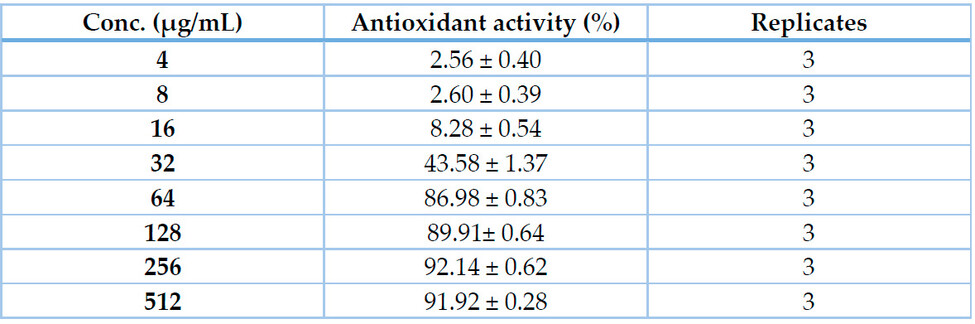
Table 2. This Antioxidant activity (% from 2 mM Ascorbate as a standard) of Acacia nilotica seed extract (4-512 μg/mL) indicated by DPPH assay.
Figure 3. Phospho-molybdenum antioxidant assay. Line graph showing the % of the phospho-molybdenum complex formation by different concentrations of Acacia nilotica seed hydromethanolic extract (4-512 μg/mL); values are means ± SEM of triplicates.
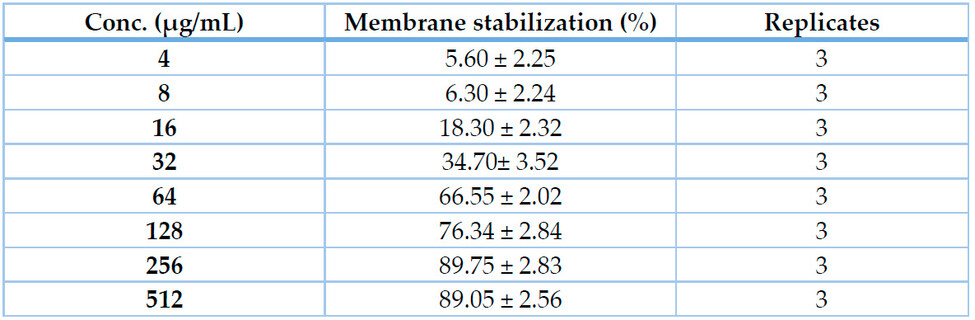
Table 3. Membrane stabilizing activity (% from 100 μM Diclofenac as a standard) of Acacia nilotica seed extract (4-512 μg/mL) indicated by HRBC-MS assay.

Figure 4. HRBC-MS assay. Line graph showing the % of the membrane stability exhibited by different concentrations of Acacia nilotica seed extract (4-512 μg/mL); values are means ± SEM of triplicates.
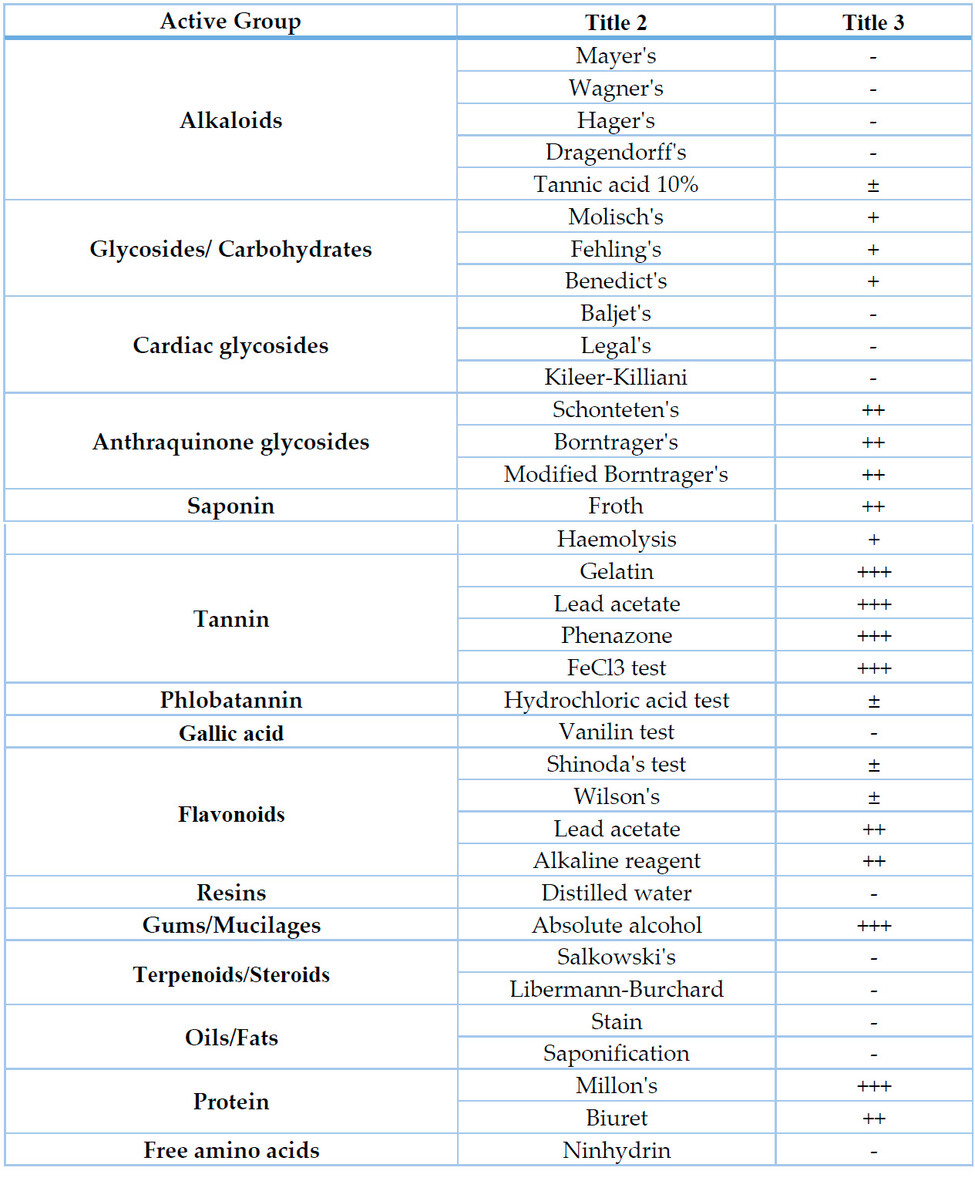
Table 4. Phytochemical analysis results of the seed extract of Acacia nilotica.
DISCUSSION
The search for new antioxidant pharmaceutical preparations from natural sources such as plants has increased over the last few decades. Antioxidants seem to play a considerable role in prophylaxis as well as therapy against reactions of un-scavenged ROS (reactive oxygen species) with cellular lipids, proteins, carbohydrates, and nucleic acids that may induce irreversible functional alterations or even complete damage to the exposed cells that are associated with numerous disease disorders 29. The current study was designed to introduce scientific evidence for the antioxidant and membrane stabilizing activities (in vitro) of the hydro-methanolic extract of the mature seeds of Acacia nilotica plant. Phytochemical analysis using classical qualitative tests was also carried out to detect the content of the active phytochemical groups that underlie these activities, if any.
The antioxidant potential of Acacia nilotica seed extract was quantified spectrophotometrically using a phospho-molybdenum complex formation assay. The present experiment demonstrated that the extract exhibited potent antioxidant activity indicated by this assay, with a maximum effect of 35.80 ± 1.35% from the used standard (Ascorbic acid 2 mM) by concentration of 512 μg/mL, while the IC50 was calculated as 100.5 μg/mL. Previous evidence has shown that medicinal plants' phenols, flavonoids, and related compounds significantly reduce MO-(VI) and phosph-MO-(V) production 30. These compounds were evidenced in Acacia nilotica seed extract using phytochemical analysis, and their presence may explain the plant's MO-(VI) scavenging activity.
The ability of Hydrogen donation by a particular substance could be determined by DPPH assay. An antioxidant that gives that proton neutralizes and, thus, decolorizes the violet color of the DPPH- solution, and the extent of color bleaching is directly related to either the concentration or potency or both of the tested antioxidants. In the present assay, the seed extract of Acacia nilotica showed significant inhibition % that was a maximum of 92.14 ± 0.62% by 256 μg/mL, compared to the standard (2 mM ascorbic acid). This result may suggest that Acacia nilotica seed extract contains active principle(s) that are (are) able to donate hydrogen to scavenge a free radical and protect against its potential damage. These principles include one or more of those tested positively using phytochemical screening, especially tannins/phenols and flavonoids.
Antioxidant findings proved in the present study may be parallel to those of 31 who reported that the extracts from leaves, bark and pods of Acacia nilotica exhibited antioxidant properties indicated by DPPH- assay and attributed such activity to the high total phenolic contents of the parts used.
The effect of Acacia nilotica seed extract on RBC membrane stability was potent and promising. The extract exhibited a concentration-dependent membrane stabilizing effect, as indicated by guarding against lysis of RBCs when challenged with hypotonic saline solution. The maximum % of membrane stabilization produced by the extract was 89.75 ± 2.83%, recorded at a concentration of 256 μg/mL, compared to the standard drug Diclofenac potassium, with an IC50 value of 41.47 μg/mL. The structure of erythrocytic membranes is analogous to that of lysosomes 32 and, thus, its stabilization and integrity-keeping potential may imply that the seed extract may be necessary in limiting the inflammatory response via inhibition of inflammatory mediators release from lysosomes and mast cells as well as cells exposed to oxidative stress and injury 33.
Qualitative phytochemical analysis of the seed extracts revealed the presence of high (+++) contents of many phytochemical groups. Tannins and flavonoids, in particular, may account for the anti-inflammatory and antioxidant potentials exhibited by the Acacia plant extract. These results partially follow those of 31 who found that other parts of the plant, including leaves, bark and pods, are rich in total phenolic compounds that were characterized and identified by liquid chromatography-tandem mass spectrometry as galloylated catechins and gallocatechin derivatives in tested extracts. Our extract, however, was negative for the gallic acid detection test.
CONCLUSIONS
It could be concluded that the extract of Acacia nilotica seeds has a strong potential for antioxidant and membrane stabilizing activities, and these potentials are attributed to their content and beneficial phytochemical constituents. The seeds of the Acacia nilotica plant, thus, could be an excellent pharmaceutical source of natural antioxidant and anti-inflammatory medicines.
Funding: This research received no external funding.
Conflicts of Interest: The author declares no conflict of interest.
REFERENCES
1. Malviya S, Rawat S, Kharia A, Verma M. Medicinal attributes of Acacia nilotica Linn.-A comprehensive review on ethnopharmacological claims. Int J Pharm Life Sci. 2011;2(6):830-7.
2. Orwa C, Mutua A, Kindt R, Jamnadass R, Simons A. Agroforestree database: a tree species reference and selection guide version 4.0. World Agroforestry Centre ICRAF, Nairobi, KE. 2009.
3. Shah BH, Safdar B, Virani SS, Nawaz Z, Saeed S, Gilani A. The antiplatelet aggregatory activity of Acacia nilotica is due to blockade of calcium influx through membrane calcium channels. Gen Pharmacol: The Vascular System. 1997;29(2):251-5.
4. Gilani A, Shaheen F, Zaman M, Janbaz K, Shah B, Akhtar M. Studies on antihypertensive and antispasmodic activities of methanol extract of Acacia nilotica pods. Phytother Res. 1999;13(8):665-9.
5. Satish S, Mohana D, Ranhavendra M, Raveesha K. Antifungal activity of some plant extracts against important seed borne pathogens of Aspergillus sp. Int J Agr Technol. 2007;3(1):109-19.
6. Bachaya HA, Iqbal Z, Khan MN, Jabbar A. Anthelmintic activity of Ziziphus nummularia (bark) and Acacia nilotica (fruit) against Trichostrongylid nematodes of sheep. J Ethnopharmacol. 2009;123(2):325-9.
7. Mohamed IET, Nur E, Abdelrahman MEN. The antibacterial, antiviral activities and phytochemical screening of some Sudanese medicinal plants. EurAsian J BioSci. 2010;4.
8. Sanni S, Thilza I, Talle M, Mohammed S, Sanni F, Okpoli L, et al. The effect of acacia nilotica pob ethyl acetate fraction on induced diarrhea in albino rats. New York Science Journal. 2010;3(8):16-20.
9. Sadiq MB, Tarning J, Aye Cho TZ, Anal AK. Antibacterial activities and possible modes of action of Acacia nilotica (L.) Del. Against multidrug-resistant Escherichia coli and salmonella. Molecules. 2017;22(1):47.
10. Sadiq MB, Tharaphan P, Chotivanich K, Tarning J, Anal AK. In vitro antioxidant and antimalarial activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. BMC Complement Altern Med. 2017;17(1):372.
11. Dafallah AA, Al-Mustafa Z. Investigation of the anti-inflammatory activity of Acacia nilotica and Hibiscus sabdariffa. Am J Chin Medicine. 1996;24(03n04):263-9.
12. Almeida R, Navarro D, Barbosa-Filho J. Plants with central analgesic activity. Phytomedicine. 2001;8(4):310-22.
13. Halliwell B, Gutteridge JM. Formation of a thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS letters. 1981;128(2):347-52.
14. Nunes XP, Silva FS, Almeida JRGdS, de Lima JT, de Araújo Ribeiro LA, Júnior LJQ, et al. Biological oxidations and antioxidant activity of natural products. Phytochemicals as nutraceuticals-Global Approaches to Their Role in Nutrition and Health: InTech; 2012.
15. Harborne A. Phytochemical methods a guide to modern techniques of plant analysis: springer science & business media; 1998.
16. Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269(2):337-41.
17. Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199-200.
18. Manzocco L, Anese M, Nicoli M. Antioxidant properties of tea extracts as affected by processing. LWT-Food Science and Technology. 1998;31(7):694-8.
19. Trnavsky K, Scherrer R, Whitehouse M. Drug effects on connective tissue metabolism. Anti-inflammatory agents. 1974;2:303-26.
20. Harborne J. Phytochemical methods, a guide to modern techniques of plant analysis, JB Harborne. Chapman London GB. 1973.
21. Claus EP, Tyler VE. Pharmacognosy. 5th ed. Philadelphia.: Lea & Febiger; 1967.
22. Balbaa SI. Chemistry of Crude Drugs-Laboratory Manual. Faculty of Pharmacy, Cairo University: Al-Shaab Printing House; 1986. 195 p.
23. Evans W, Evans D. Trease and Evans Pharmacognosy. 15 ed. Singapore: Sanders Co. Ltd.; 2002. 585 p.
24. Ramakrishnan S. Textbook of Medical Biochemistry: Orient Longman; 2004.
25. Whistler RL, BeMiller JN. Industrial Gums: Polysaccharides and Their Derivatives. 3rd ed. UK: Academic Press; 1993.
26. Kokate CK. Practical pharmacognosy. New Delhi, India: Vallabh Prakashan; 2008.
27. Rasch E, SWIFT H. Microphotometric analysis of the cytochemical Millon reaction. J Histochem Cytochem. 1960;8(1):4-17.
28. Yasuma A, Ichikawa T. Ninhydrin-Schiff and alloxan-Schiff staining. J Lab Clin Med. 1953;41(2):296-9.
29. D'Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nature reviews Molecular cell biology. 2007;8(10):813-24.
30. Sharififar F, Dehghn-Nudeh G, Mirtajaldini M. Major flavonoids with antioxidant activity from Teucrium polium L. Food Chem. 2009;112(4):885-8.
31. Sadiq MB, Hanpithakpong W, Tarning J, Anal AK. Screening of phytochemicals and in vitro evaluation of antibacterial and antioxidant activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. Industrial Crops and Products. 2015;77:873-82.
32. Chou CT. The Anti-inflammatory Effect of an Extract of Tripterygium wilfordii Hook F on Adjuvant‐induced Paw Oedema in Rats and Inflammatory Mediators Release. Phytother Res. 1997;11(2):152-4.
33. Murugesh N, Vembar S, Damodaran C. Studies on erythrocyte membrane IV: in vitro haemolytic activity of oleander extract. Toxicol Lett. 1981;8(1):33-8.
Received: 9 October 2023/ Accepted: 15 January 2024 / Published: 15 February 2024
Citation: Abubakr El-Mahmoudy. Phytochemical analysis underlying membrane stabilization and antioxidant promising potentials of Acacia nilotica seed extract. Bionatura Journal 2024; 1 (1) 31. http://dx.doi.org/10.21931/BJ/2024.01.01.31
Additional information Correspondence should be addressed to a.elmahmoudy@fvtm.bu.edu.eg
Peer review information. Bionatura Journal thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Publisher's Note: Bionatura Journal stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2024 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
